Electrospun Composites Made of Reduced Graphene Oxide and Polyacrylonitrile-Based Activated Carbon Nanofibers (rGO/ACNF) for Enhanced CO2 Adsorption
Abstract
:1. Introduction
2. Materials and Methods
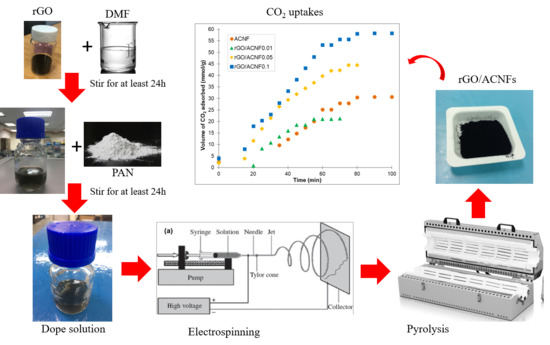
2.1. Synthesis of Reduced Graphene Oxide (rGO)
2.2. Preparation of Activated Carbon Nanofibers Nanocomposites (gACNFs)
2.3. Characterization of Materials
2.4. CO2 Adsorption Experiments
2.5. Kinetic Studies
3. Results and Discussion
3.1. Properties of Materials
3.2. CO2 Adsorption Performance
3.3. Adsorption Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Acevedo, E.R.; Cortés, F.B.; Franco, C.A.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Fierro, V.; Celzard, A.; Schaefer, S.; Molina, A.C. An Enhanced Carbon Capture and Storage Process (e-CCS) Applied to Shallow Reservoirs Using Nanofluids Based on Nitrogen-Rich Carbon Nanospheres. Materials 2019, 12, 2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.-G.; Liu, Y.-F.; Wu, X.-X.; Cai, J. Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. New Carbon Mater. 2019, 34, 247–257. [Google Scholar] [CrossRef]
- Bains, P.; Psarras, P.; Wilcox, J. CO2 capture from the industry sector. Prog. Energy Combust. Sci. 2017, 63, 146–172. [Google Scholar] [CrossRef]
- Acevedo, E.R.; Franco, C.A.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Cortés, F.B. Biomass-Derived Carbon Molecular Sieves Applied to an Enhanced Carbon Capture and Storage Process (e-CCS) for Flue Gas Streams in Shallow Reservoirs. Nanomaterials 2020, 10, 980. [Google Scholar] [CrossRef]
- Carbon Cycle Greenhouse Gases, Mouna Loa Observatory. 2020. Available online: https://www.esrl.noaa.gov/gmd/ccgg/ (accessed on 17 August 2020).
- Willard, B. CO2—Why 450 ppm Is Dangerous and 350 ppm Is Safe. 2014. Available online: https://sustainabilityadvantage.com/2014/01/07/co2-why-450-ppm-is-dangerous-and-350-ppm-is-safe/ (accessed on 20 July 2020).
- Le Quéré, C.; Jackson, R.B.; Jones, M.W.; Smith, A.J.P.; Abernethy, S.; Andrew, R.; De-Gol, A.J.; Willis, D.R.; Shan, Y.; Canadell, J.G.; et al. Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nat. Clim. Chang. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.P.; Bashir, S.; Liu, J. Chapter 7- Carbon Capture and Storage. In Advanced Nanomaterials and Their Applications in Renewable Energy; Liu, J., Bashir, S., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2015; pp. 329–366. [Google Scholar]
- Abbasi, A.; Nasef, M.M.; Babadi, F.E.; Faridi-Majidi, R.; Takeshi, M.; Abouzari-Lotf, E.; Choong, T.; Somwangthanaroj, A.; Kheawhom, S. Carbon Dioxide Adsorption on Grafted Nanofibrous Adsorbents Functionalized Using Different Amines. Front. Energy Res. 2019, 7, 1–14. [Google Scholar] [CrossRef]
- Romano, M.C.; Anantharaman, R.; Arasto, A.; Ozcan, D.C.; Ahn, H.; Dijkstra, J.; Carbo, M.; Boavida, D. Application of Advanced Technologies for CO2 Capture From Industrial Sources. Energy Procedia 2013, 37, 7176–7185. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Kumar, E.A.; Emadabathuni, A.K. Measurement and analysis of adsorption isotherms of CO2 on activated carbon. Appl. Therm. Eng. 2016, 97, 77–86. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Serna-Guerrero, R.; Sayari, A. Adsorption of CO2 from dry gases on MCM-41 silica at ambient temperature and high pressure. 1: Pure CO2 adsorption. Chem. Eng. Sci. 2009, 64, 3721–3728. [Google Scholar] [CrossRef]
- Hauchhum, L.; Mahanta, P. Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. Int. J. Energy Environ. Eng. 2014, 5, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Al-Janabi, N.; Hill, P.; Torrente-Murciano, L.; Garforth, A.; Gorgojo, P.; Siperstein, F.; Fan, X. Mapping the Cu-BTC metal—Organic framework (HKUST-1) stability envelope in the presence of water vapour for CO2 adsorption from flue gases. Chem. Eng. J. 2015, 281, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Al-Janabi, N.; Deng, H.; Borges, J.; Liu, X.; Garforth, A.; Siperstein, F.R.; Fan, X. A facile post-synthetic modification method to improve hydrothermal stability and CO2 selectivity of CyBTC metal-organic framework. Ind. Eng. Chem. Res. 2016, 55, 7941–7949. [Google Scholar] [CrossRef] [Green Version]
- Olijire, A.A. Synthesis chemistry of metal-organic frameworks for CO2 capture andconversion for sustainable energy future. Renew. Sustain. Energy Rev. 2018, 92, 570–607. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Hsu, W.-L.; Lin, S.-Y.; Juang, R.-S. Enhanced CO2 Adsorption on Activated Carbon Fibers Grafted with Nitrogen-Doped Carbon Nanotubes. Materials 2017, 10, 511. [Google Scholar] [CrossRef]
- Bhatta, L.K.G.; Bhatta, U.M.; Venkatesh, K. Metal Oxides for Carbon Dioxide Capture. Sustain. Agric. Rev. 2019, 38, 63–83. [Google Scholar] [CrossRef]
- Rodríguez-García, S.; Santiago, R.; López-Díaz, D.; Merchán, M.D.; Velázquez, M.M.; Fierro, J.L.G.; Palomar, J. Role of the Structure of Graphene Oxide Sheets on the CO2 Adsorption Properties of Nanocomposites Based on Graphene Oxide and Polyaniline or Fe3O4-Nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 12464–12473. [Google Scholar] [CrossRef]
- Pellerano, M.; Pré, P.; Kacem, M.; Delebarre, A. CO2 capture by adsorption on activated carbons using pressure modulation. Energy Procedia 2009, 1, 647–653. [Google Scholar] [CrossRef] [Green Version]
- El-Sharkawy, I.I.; Mansour, M.H.; Awad, M.M.; El-Ashry, R. Investigation of Natural Gas Storage through Activated Carbon. J. Chem. Eng. Data 2015, 60, 3215–3223. [Google Scholar] [CrossRef]
- Othman, F.E.C.; Yusof, N.; Hasbullah, H.; Jaafar, J.; Ismail, A.F.; Nasri, N.S. Physicochemical properties and methane adsorption performance of activated carbon nanofibers with different types of metal oxides. Carbon Lett. 2017, 24, 82–89. [Google Scholar]
- Lee, H.-M.; Kim, H.-G.; An, K.-H.; Kim, B.-J. Effects of pore structures on electrochemical behaviors of polyacrylonitrile-based activated carbon nanofibers by carbon dioxide activation. Carbon Lett. 2014, 15, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Tavanai, H.; Jalili, R.; Morshed, M.; Jalili, R. Effects of fiber diameter and CO2 activation temperature on the pore characteristics of polyacrylonitrile based activated carbon nanofibers. Surf. Interface Anal. 2009, 41, 814–819. [Google Scholar] [CrossRef]
- Othman, F.E.C.; Yusof, N.; Ismail, A.F.; Jaafar, J.; Salleh, W.N.W.; Aziz, F. Preparation and characterization of polyacrylonitrile-based activated carbon nanofibers/graphene (gACNFs) composite synthesized by electrospinning. AIP Adv. 2020, 10, 055117. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Carbon dioxide adsorption in graphene sheets. AIP Adv. 2011, 1, 32152. [Google Scholar] [CrossRef]
- Takeuchi, K.; Yamamoto, S.; Hamamoto, Y.; Shiozawa, Y.; Tashima, K.; Fukidome, H.; Koitaya, T.; Mukai, K.; Yoshimoto, S.; Suemitsu, M.; et al. Adsorption of CO2 on Graphene: A Combined TPD, XPS, and vdW-DF Study. J. Phys. Chem. C 2017, 121, 2807–2814. [Google Scholar] [CrossRef]
- Wang, Z.; Ciacchi, L.C.; Wei, G. Recent Advances in the Synthesis of Graphene-Based Nanomaterials for Controlled Drug Delivery. Appl. Sci. 2017, 7, 1175. [Google Scholar] [CrossRef] [Green Version]
- Alghamdi, A.A.; Alshahrani, A.F.; Khdary, N.H.; Alharthi, F.A.; Alattas, H.A.; Adil, S.F. Enhanced CO2 Adsorption by Nitrogen-Doped Graphene Oxide Sheets (N-GOs) Prepared by Employing Polymeric Precursors. Materials 2018, 11, 578. [Google Scholar] [CrossRef] [Green Version]
- Yoo, M.J.; Park, H. Effect of hydrogen peroxide on properties of graphene oxide in Hummers method. Carbon 2019, 141, 515–522. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (rGO)*. Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Othman, F.E.C.; Yusof, N.; Hasbullah, H.; Othman, M.H.D.; Ismail, A.F.; Abdullah, N.; Nordin, N.A.H.M.; Aziz, F.; Salleh, W.N.W. Polyacrylonitrile/magnesium oxide-based activated carbon nanofibers with well-developed microporous structure and their adsorption performance for methane. J. Ind. Eng. Chem. 2017, 51, 281–287. [Google Scholar] [CrossRef]
- Nasri, N.S.; Hamza, U.D.; Ismail, S.N.; Ahmed, M.M.; Mohsin, R. Assessment of porous carbons derived from sustainable palm solid waste for carbon dioxide capture. J. Clean. Prod. 2014, 71, 148–157. [Google Scholar] [CrossRef]
- Stevens, L.; Williams, K.; Han, W.Y.; Drage, T.C.; Snape, C.; Wood, J.; Wang, J. Preparation and CO2 adsorption of diamine modified montmorillonite via exfoliation grafting route. Chem. Eng. J. 2013, 215, 699–708. [Google Scholar] [CrossRef]
- Lopes, E.C.; Dos Anjos, F.S.; Vieira, E.F.; Cestari, A.R. An alternative Avrami equation to evaluate kinetic parameters of the interaction of Hg(II) with thin chitosan membranes. J. Colloid Interface Sci. 2003, 263, 542–547. [Google Scholar] [CrossRef]
- Romero, J.R.G.; Moreno-Piraján, J.C.; Giraldo, L. Kinetic and Equilibrium Study of the Adsorption of CO2 in Ultramicropores of Resorcinol-Formaldehyde Aerogels Obtained in Acidic and Basic Medium. J. Carbon Res. C 2018, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-Y.; Park, S.-J. Determination of the optimal pore size for improved CO2 adsorption in activated carbon fibers. J. Colloid Interface Sci. 2013, 389, 230–235. [Google Scholar] [CrossRef]
- Sing, K.S.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Liu, H.; Ding, W.; Lei, S.; Tian, X.; Zhou, F. Selective adsorption of CH4/N2 on Ni-based MOF/SBA-15 composite materials. J. Nanomater. 2019, 9, 149, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Nasrollahzadeh, M.; Babaei, F.; Fakhri, P.; Jaleh, B. Synthesis, characterization, structural, optical properties and catalytic activity of reduced graphene oxide/copper nanocomposites. RSC Adv. 2015, 5, 10782–10789. [Google Scholar] [CrossRef]
- Kim, D.W.; Jung, D.W.; Adelodun, A.A.; Jo, Y.M. Evaluation of CO2 adsorption capacity of electrospun carbon fibers with thermala and chemical activation. J. Appl. Polym. Sci. 2017, 134, 45534. [Google Scholar] [CrossRef]
- Gayathri, S.; Jayabal, P.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of few layer graphene by direct exfoliation of graphite and a Raman spectroscopic study. AIP Adv. 2014, 4, 27116. [Google Scholar] [CrossRef]
- Frank, O.; Mohr, M.; Maultzsch, J.; Thomsen, C.; Riaz, I.; Jalil, R.; Novoselov, K.S.; Tsoukleri, G.; Parthenios, J.; Papagelis, K.; et al. Raman 2D-Band Splitting in Graphene: Theory and Experiment. ACS Nano 2011, 5, 2231–2239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.M.; Kim, S.H.; Jeong, B.G.; Jo, S.M.; Lee, K.B. Development of porous carbon nanofibers from electrospun polyvinylidene fluoride for CO2 capture. RSC Adv. 2014, 4, 58956–58963. [Google Scholar] [CrossRef]
- Badawy, S.M.; Dessouki, A.M. Cross-Linked Polyacrylonitrile Prepared by Radiation-Induced Polymerization Technique. J. Phys. Chem. B 2003, 107, 11273–11279. [Google Scholar] [CrossRef]
- Jyothi, N.K.; Kumar, K.V.; Murthy, P.N. FTIR, XRD and DC conductivity studies of proton conducting gel polymer electrolytes based on polyacrylonitrile (PAN). Int. J. ChemTech Res. 2014, 6, 5214–5219. [Google Scholar]
- Lee, H.-M.; Kang, H.-R.; An, K.H.; Kim, H.-G.; Kim, B.-J. Comparative studies of porous carbon nanofibers by various activation methods. Carbon Lett. 2013, 14, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Ouassim, B.; Fouad, G.; Arunabh, G.; Ouafae, A.; Tarik, C. Excellent CO2 capture by ultra-high microporous activated carbon made out from Natural coal. Chem. Eng. Technol. 2020. under review. [Google Scholar]
- Guo, J.; Morris, J.R.; Ihm, Y.; Contescu, C.I.; Gallego, N.C.; Duscher, G.; Pennycook, S.J.; Chisholm, M.F. Topological Defects: Origin of Nanopores and Enhanced Adsorption Performance in Nanoporous Carbon. Small 2012, 8, 3283–3288. [Google Scholar] [CrossRef]
- Bin Ali, A.; Renz, F.; Koch, J.; Tegenkamp, C.; Sindelar, R. Graphene Nanoplatelet (GNPs) Doped Carbon Nanofiber (CNF) System: Effect of GNPs on the Graphitic Structure of Creep Stress and Non-Creep Stress Stabilized Polyacrylonitrile (PAN). Nanomaterials 2020, 10, 351. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.-M.; Zhang, Y.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Ahmad, M.; Wang, J.; Xu, J.; Zhang, Q.; Zhang, B. Magnetic tubular carbon nanofibers as efficient Cu(II) ion adsorbent from wastewater. J. Clean. Prod. 2020, 252, 119825. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular Bamboo-Derived Activated Carbon for High CO2Adsorption: The Dominant Role of Narrow Micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- Li, K.; Tian, S.; Wang, J.; Jiang, J.; Chen, X.; Yan, F. Pine cone shell-based activated carbon used for CO2 adsorption. J. Mater. Chem. A 2016, 4, 5223–5234. [Google Scholar] [CrossRef]
- Chen, L.; Watanabe, T.; Kanoh, H.; Hata, K.; Ohba, T. Cooperative CO2 adsorption promotes high CO2 adsorption density over wide optimal nanopore range. Adsorpt. Sci. Technol. 2017, 36, 625–639. [Google Scholar] [CrossRef] [Green Version]
- Ullah, S.; Shariff, A.M.; Bustam, M.A.; Elkhalifah, A.E.I.; Murshid, G.; Riaz, N.; Shimekit, B. Modified MIL-53 with multi-wall carbon nanotubes and nano fibers on CO2 adsorption. Appl. Mech. Mater. 2014, 625, 870–873. [Google Scholar] [CrossRef]
- Ojeda-López, R.; Esparza-Schulz, M.; Pérez-Hermosillo, I.J.; Hernández-Gordillo, A.; Domínguez-Ortiz, A. Improve in CO2 and CH4 Adsorption Capacity on Carbon Microfibers Synthesized by Electrospinning of PAN. Fibers 2019, 7, 81. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.-Y.; Park, S.-J. Effect of heat treatment on CO2 adsorption of KOH-activated graphite nanofibers. J. Colloid Interface Sci. 2010, 352, 498–503. [Google Scholar] [CrossRef]
- Yuan, H.; Meng, L.-Y.; Park, S.-J. KOH-activated graphite nanofibers as CO2 adsorbents. Carbon Lett. 2016, 19, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.-J.; Wang, J.-S.; Liu, Y.; Li, X.; Zeng, G.-M.; Bao, Z.-L.; Zeng, X.-X.; Chen, A.; Long, F. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2011, 185, 306–314. [Google Scholar] [CrossRef]
- Martín, C.; Sweatman, M.; Brandani, S.; Fan, X. Wet impregnation of a commercial low cost silica using DETA for a fast post-combustion CO2 capture process. Appl. Energy 2016, 183, 1705–1721. [Google Scholar] [CrossRef] [Green Version]
- Berger, A.H.; Bhown, A.S. Comparing physisorption and chemisorption solid sorbents for use separating CO2 from flue gas using temperature swing adsorption. Energy Procedia 2011, 4, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Culp, J.T.; Smith, M.R.; Bittner, E.; Bockrath, B. Hysteresis in the Physisorption of CO2 and N2 in a Flexible Pillared Layer Nickel Cyanide. J. Am. Chem. Soc. 2008, 130, 12427–12434. [Google Scholar] [CrossRef]









| Samples | SBET (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Vmicro/Vtotal (%) | |
|---|---|---|---|---|---|---|
| Before Activation | NF | 17 | 0.14 | 0 | 0 | 0 |
| rGO/NF0.01 | 10 | 0.07 | 0 | 0 | 0 | |
| rGO/NF0.05 | 8 | 0.05 | 0 | 0 | 0 | |
| rGO/NF0.1 | 32 | 0.30 | 0 | 0 | 0 | |
| After Activation | Activated carbon nanofibers (ACNF) | 139 | 0.09 | 0.06 | 0.03 | 67 |
| rGO/ACNF0.01 | 92 | 0.07 | 0.04 | 0.03 | 57 | |
| rGO/ACNF0.05 | 233 | 0.14 | 0.09 | 0.05 | 64 | |
| rGO/ACNF0.1 | 373 | 0.22 | 0.15 | 0.07 | 68 |
| Sample | CO2 Uptakes (mmol/g) | ||
|---|---|---|---|
| 5 bar | 10 bar | 15 bar | |
| ACNF | 24 | 27 | 31 |
| rGO/ACNF0.01 | 17 | 20 | 21 |
| rGO/ACNF0.05 | 34 | 36 | 44 |
| rGO/ACNF0.1 | 41 | 43 | 58 |
| Materials | SBET (m2/g) | Vmicro (cm3/g) | Vol. of CO2 Adsorbed (mmol/g) | Temp; Pressure | Ref. |
|---|---|---|---|---|---|
| rGO/ACNF0.1 | 373 | 0.22 | 41 | 25 °C; 5 bar | This work |
| 43 | 25 °C; 10 bar | ||||
| 58 | 25 °C; 15 bar | ||||
| PVDF-CNFs | 1065 | 0.61 | 3.1 | 30 °C; 1 atm | Hong et al., 2014 [58] |
| CNF-SnO2 | 434 | 0.20 | 2.6 | 25 °C; 1 atm | Ali et al., 2020 [54] |
| CNF-MIL-53 | 140 | - | 1.35 | 25 °C; 1 bar | Ullah et al., 2014 [59] |
| PAN-CMFs | 966 | - | 2.9 | 25 °C; 1 bar | Ojeda-Lopez et al., 2019 [60] |
| Graphite NFs | 567 | 0.27 | 59.2 mg/g | 25 °C; 1 atm | Meng and Park, 2010 [61] |
| Graphite NFs | 966 | 0.25 | 70.8 mg/g | 25 °C; 1 atm | Yuan et al., 2016 [62] |
| Sample | Pressure (bar) | qe,exp (mmol/g) | Pseudo-First Order | Pseudo-Second Order | ||
|---|---|---|---|---|---|---|
| k1 | R2 | k2 | R2 | |||
| ACNF | 5 | 24 | 0.1036 | 0.6337 | 0.1397 | 0.9645 |
| rGO/ACNF0.1 | 41 | 0.0865 | 0.7566 | 0.1435 | 0.9825 | |
| ACNF | 10 | 27 | 0.1217 | 0.7595 | 0.1902 | 0.6563 |
| rGO/ACNF0.1 | 46 | 0.0941 | 0.7667 | 0.0531 | 0.9127 | |
| ACNF | 15 | 31 | 0.1114 | 0.7266 | 0.0319 | 0.3897 |
| rGO/ACNF0.1 | 58 | 0.0716 | 0.7861 | 0.0697 | 0.2493 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che Othman, F.E.; Yusof, N.; González-Benito, J.; Fan, X.; Ismail, A.F. Electrospun Composites Made of Reduced Graphene Oxide and Polyacrylonitrile-Based Activated Carbon Nanofibers (rGO/ACNF) for Enhanced CO2 Adsorption. Polymers 2020, 12, 2117. https://doi.org/10.3390/polym12092117
Che Othman FE, Yusof N, González-Benito J, Fan X, Ismail AF. Electrospun Composites Made of Reduced Graphene Oxide and Polyacrylonitrile-Based Activated Carbon Nanofibers (rGO/ACNF) for Enhanced CO2 Adsorption. Polymers. 2020; 12(9):2117. https://doi.org/10.3390/polym12092117
Chicago/Turabian StyleChe Othman, Faten Ermala, Norhaniza Yusof, Javier González-Benito, Xiaolei Fan, and Ahmad Fauzi Ismail. 2020. "Electrospun Composites Made of Reduced Graphene Oxide and Polyacrylonitrile-Based Activated Carbon Nanofibers (rGO/ACNF) for Enhanced CO2 Adsorption" Polymers 12, no. 9: 2117. https://doi.org/10.3390/polym12092117