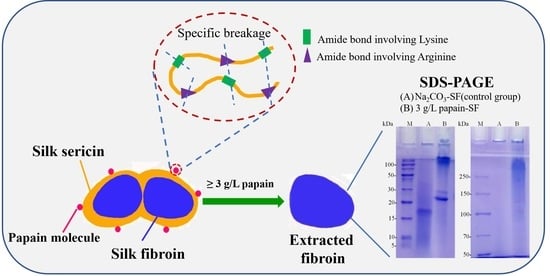
High Molecular Weight Silk Fibroin Prepared by Papain Degumming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Silk Degumming with Different Concentrations of Papain
2.3. Weight Loss Measurements
2.4. Picric Acid and Carmine Staining (PACS) Assay
2.5. Morphological Characterization of Degummed Silks
2.6. Tensile Properties
2.7. Preparation of Regenerated SF Solution and SF Solids
2.8. Amino Acid Analysis
2.9. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3. Results and Discussion
3.1. Degumming Ratio
3.2. PACS Assay
3.3. Morphological Observation of Degummed Silk Fibers
3.4. Effect of Degumming Method on the Mechanical Performance of Silk Fibers
3.5. Amino Acid Analysis
3.6. Influence of Degumming Methods on the MWD
3.7. Potential Degumming Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cranford, S.W.; Tarakanova, A.; Pugno, N.M.; Buehler, M.J. Nonlinear material behaviour of spider silk yields robust webs. Nature 2012, 482, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Li, X.; Gao, E.; Jian, M.; Xia, K.; Wang, Q.; Xu, Z.; Ren, T.; Zhang, Y. Carbonized silk fabric for ultrastretchable, highly sensitive, and wearable strain sensors. Adv. Mater. 2016, 28, 6640–6648. [Google Scholar] [CrossRef] [PubMed]
- Omenetto, F.G.; Kaplan, D.L. New opportunities for an ancient material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, F.; Porter, D. Silks as ancient models for modern polymers. Polymer 2009, 50, 5623–5632. [Google Scholar] [CrossRef] [Green Version]
- Mahmoodi, N.M.; Arami, M. Numerical finite volume modeling of dye decolorization using immobilized titania nanophotocatalysis. Chem. Eng. J. 2009, 146, 189–193. [Google Scholar] [CrossRef]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.S.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Teuschl, A.H.; Van Griensven, M.; Redl, H. Sericin removal from raw Bombyx mori silk scaffolds of high hierarchical order. Tissue Eng. Part C Methods 2014, 20, 431–439. [Google Scholar] [CrossRef]
- Wray, L.S.; Hu, X.; Gallego, J.; Georgakoudi, I.; Omenetto, F.G.; Schmidt, D.; Kaplan, D.L. Effect of processing on silk-based biomaterials: Reproducibility and biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 89–101. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; Yang, Y.; Shao, Z. Effect of various dissolution systems on the molecular weight of regenerated silk fibroin. Biomacromolecules 2013, 14, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, M.K.; Lee, K.H.; Nho, S.K.; Han, M.S.; Um, I.C. Effect of degumming methods on structural characteristics and properties of regenerated silk. Int. J. Biol. Macromol. 2017, 104, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Ki, C.S.; Oh, H.; Lee, K.H.; Um, I.C. Molecular weight distribution and solution properties of silk fibroins with different dissolution conditions. Int. J. Biol. Macromol. 2012, 51, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Song, D.W.; Kim, M.J.; Ryu, S.J.; Um, I.C.; Ki, C.S.; Park, Y.H. Effect of silk fibroin molecular weight on physical property of silk hydrogel. Polymer 2016, 90, 26–33. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Q.; Ming, J.; Dou, H.; Liu, Z.; Zuo, B.; Qin, M.; Li, F.; Kaplan, D.L.; Zhang, X. Silk dissolution and regeneration at the nanofibril scale. J. Mater. Chem. B 2014, 2, 3879–3885. [Google Scholar] [CrossRef]
- Gupta, D.; Agrawal, A.; Chaudhary, H.; Gulrajani, M.; Gupta, C. Cleaner process for extraction of sericin using infrared. J. Clean. Prod. 2013, 52, 488–494. [Google Scholar] [CrossRef]
- Moazami, A.; Montazer, M.; Rashidi, A.; Rahimi, M.K. Antibacterial properties of raw and degummed silk with nanosilver in various conditions. J. Appl. Polym. Sci. 2010, 118, 253–258. [Google Scholar] [CrossRef]
- Dou, H.; Zuo, B. Effect of sodium carbonate concentrations on the degumming and regeneration process of silk fibroin. J. Text. Inst. 2014, 106, 311–319. [Google Scholar] [CrossRef]
- Nultsch, K.; Bast, L.K.; Näf, M.; Yakhlifi, S.E.; Bruns, N.; Germershaus, O. Effects of silk degumming process on physicochemical, tensile, and optical properties of regenerated silk fibroin. Macromol. Mater. Eng. 2018, 303, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pantano, M.F.; Berardo, A.; Pugno, N.M. Tightening slip knots in raw and degummed silk to increase toughness without losing strength. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Rigueiro, J.; Elices, M.; Llorca, J.; Viney, C. Effect of degumming on the tensile properties of silkworm (Bombyx mori) silk fiber. J. Appl. Polym. Sci. 2002, 84, 1431–1437. [Google Scholar] [CrossRef]
- Wang, F.; Cao, T.; Zhang, Y. Effect of silk protein surfactant on silk degumming and its properties. Mater. Sci. Eng. C 2015, 55, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.P.; Wang, H.; Lau, K.T.; Lee, J.H.; Hui, D. Interfacial bonding and degumming effects on silk fibre/polymer biocomposites. Compos. Part B Eng. 2012, 43, 2801–2812. [Google Scholar] [CrossRef]
- Ho, M.P.; Wang, H.; Lau, K. Effect of degumming time on silkworm silk fibre for biodegradable polymer composites. Appl. Surf. Sci. 2012, 258, 3948–3955. [Google Scholar] [CrossRef]
- Arami, M.; Rahimi, S.; Mivehie, L.; Mazaheri, F.; Mahmoodi, N.M. Degumming of persian silk with mixed proteolytic enzymes. J. Appl. Polym. Sci. 2007, 106, 267–275. [Google Scholar] [CrossRef]
- Suwannaphan, S.; Fufeungsombut, E.; Promboon, A.; Chim-Anage, P. A serine protease from newly isolated Bacillus sp. for efficient silk degumming, sericin degrading and colour bleaching activities. Int. Biodeter. Biodegr. 2017, 117, 141–149. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, M.; Kim, S. Biological degumming of silk fabrics with proteolytic enzymes. J. Nat. Fibers 2016, 13, 629–639. [Google Scholar]
- Freddi, G.; Mossotti, R.; Innocenti, R. Degumming of silk fabric with several proteases. J. Biotechnol. 2003, 106, 101–112. [Google Scholar] [CrossRef]
- More, S.V.; Khandelwal, H.B.; Joseph, M.A.; Laxman, R.S. Enzymatic degumming of silk with microbial proteases. J. Nat. Fibers 2013, 10, 98–111. [Google Scholar] [CrossRef]
- More, S.V.; Chavan, S.; Prabhune, A.A. Silk degumming and utilization of silk sericin by hydrolysis using alkaline protease from Beauveria Sp. (MTCC 5184): A green approach. J. Nat. Fibers 2017, 15, 373–383. [Google Scholar] [CrossRef]
- Gulrajani, M.L.; Agarwal, R.; Grover, A.; Suri, M. Degumming of silk with lipase and protease. Indian J. Fibre. Text. Res. 2000, 25, 69–74. [Google Scholar]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of beta-sheet crystals in silk. Nat. Mater. 2010, 9, 359–367. [Google Scholar] [CrossRef]
- Braun, F. Modelling self assembly of natural silk solution. Int. J. Biol. Macromol. 2003, 32, 59–65. [Google Scholar] [CrossRef]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Ude, A.U.; Eshkoor, R.A.; Zulkifili, R.; Ariffin, A.K.; Dzuraidah, A.W.; Azhari, C.H. Bombyx mori silk fibre and its composite: A review of contemporary developments. Mater. Design. 2014, 57, 298–305. [Google Scholar] [CrossRef]
- Alpay, P.; Uygun, D.A. Usage of immobilized papain for enzymatic hydrolysis of proteins. J. Mol. Catal. B Enzym. 2015, 111, 56–63. [Google Scholar] [CrossRef]
- Azmi, N.H.M. The Extraction of Amino Acids from Papaya Leaves using Supercritical Carbon Dioxide with Water. Ph.D. Thesis, University Malaysia Pahang, Pekan, Pahang, 2010. [Google Scholar]
- Lowe, G. The structure and mechanism of action of papain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1970, 257, 237–248. [Google Scholar]
- Bayramoglu, G.; Senkal, B.F.; Yilmaz, M.; Arica, M.Y. Immobilization and stabilization of papain on poly(hydroxyethyl methacrylate-ethylenglycol dimethacrylate) beads grafted with epoxy functional polymer chains via surface-initiated-atom transfer radical polymerization (SI-ATRP). Bioresour. Technol. 2011, 102, 9833–9837. [Google Scholar] [CrossRef]
- Vyas, S.K.; Shukla, S.R. Comparative study of degumming of silk varieties by different techniques. J. Text. Inst. 2015, 107, 191–199. [Google Scholar] [CrossRef]
- Padaki, N.V.; Das, B.; Thirumalesh, R.M. Enzyme applications in silk processing. Adv. Silk Sci. Tech. 2015, 6, 111–120. [Google Scholar]
- Nakpathom, M.; Somboon, B.; Narumol, N. Papain enzymatic degumming of Thai Bombyx mori silk fibers. J. Microsc. 2009, 23, 142–146. [Google Scholar]
- Feng, Y.; Li, X.; Li, M.; Ye, D.; Zhang, Q.; You, R.; Xu, W. Facile preparation of biocompatible silk fibroin/cellulose nanocomposite films with high mechanical performance. ACS Sustain. Chem. Eng. 2017, 5, 6227–6236. [Google Scholar] [CrossRef]
- Zhou, G.; Shao, Z.; Knight, D.P.; Yan, J.; Chen, X. Silk fibers extruded artificially from aqueous solution of regenerated bombyx mori silk fibroin are tougher than their natural counterparts. Adv. Mater. 2009, 21, 366–370. [Google Scholar] [CrossRef]
- You, R.; Zhang, Y.; Liu, Y.; Liu, G.; Li, M. The degradation behavior of silk fibroin derived from different ionic liquid solvents. Nat. Sci. 2013, 5, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins 2001, 44, 119–122. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Z.; Wang, C.; Chen, X.; Xu, H.; Lu, Q.; Kaplan, D.L. Bioactive silk hydrogels with tunable mechanical properties. J. Mater. Chem. B 2018, 6, 2739–2746. [Google Scholar] [CrossRef]
- Elahi, M.F.; Guan, G.; Wang, L.; King, M.W. Improved hemocompatibility of silk fibroin fabric using layer-by-layer polyelectrolyte deposition and heparin immobilization. J. Appl. Polym. Sci. 2014, 131, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, Y.; Carr, A.J.; Triffitt, J.T.; Sabokbar, A.; Xia, Z. Improved human tenocyte proliferation and differentiation in vitro by optimized silk degumming. Biomed. Mater. 2011, 6, 035010. [Google Scholar] [CrossRef] [Green Version]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Lawrence, B.D.; Omenetto, F.; Chui, K.; Kaplan, D.L. Processing methods to control silk fibroin film biomaterial features. J. Mater. Sci. 2008, 43, 6967–6985. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhang, Y.Q. Effect of regeneration of liquid silk fibroin on its structure and characterization. Soft Matter 2013, 9, 138–145. [Google Scholar] [CrossRef]
- Allardyce, B.J.; Rajkhowa, R.; Dilley, R.J.; Atlas, M.D.; Kaur, J.; Wang, X. The impact of degumming conditions on the properties of silk films for biomedical applications. Text. Res. J. 2016, 86, 275–287. [Google Scholar] [CrossRef]
- Yamada, H.; Nakao, H.; Takasu, Y.; Tsubouchi, K. Preparation of undegraded native molecular fibroin solution from silkworm cocoons. Mater. Sci. Eng. C 2001, 14, 41–46. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Yang, H.; Li, W.; Li, C. Effect of silk degumming on the structure and properties of silk fibroin. J. Text. Inst. 2018, 110, 134–140. [Google Scholar] [CrossRef]
- Gulrajani, M.L. Degumming of silk. Rev. Prog. Color. Relat. Top. 1992, 22, 79–89. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, H.; Wang, C.; Wu, L.; Huang, J.; Guo, C. Tensile behavior and morphology of differently degummed silkworm (Bombyx mori) cocoon silk fibres. Mater. Lett. 2006, 60, 919–925. [Google Scholar] [CrossRef]
- Perez-Rigueiro, J.; Viney, C.; Llorca, J.; Elices, M. Mechanical properties of silkworm silk in liquid media. Polymer 2000, 41, 8433–8439. [Google Scholar] [CrossRef]







| Sample | Breaking Stress (cN/dtex) | Breaking Strain (%) | Young’s Modulus (cN/dtex) |
|---|---|---|---|
| Non-degummed silk | 3.4 ± 0.3 | 25.2 ± 4.7 | 0.8 ± 0.1 |
| 3.0 g/L papain-silk | 2.7 ± 0.2 ** | 16.5 ± 2.2 * | 0.7 ± 0.1 * |
| 0.5 g/L Na2CO3-silk | 1.5 ± 0.2 | 8.7 ± 2.2 | 0.4 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Lin, J.; Niu, L.; Wang, Y.; Cheng, Z.; Sun, X.; Li, M. High Molecular Weight Silk Fibroin Prepared by Papain Degumming. Polymers 2020, 12, 2105. https://doi.org/10.3390/polym12092105
Feng Y, Lin J, Niu L, Wang Y, Cheng Z, Sun X, Li M. High Molecular Weight Silk Fibroin Prepared by Papain Degumming. Polymers. 2020; 12(9):2105. https://doi.org/10.3390/polym12092105
Chicago/Turabian StyleFeng, Yanfei, Jiaming Lin, Longxing Niu, Ying Wang, Zhiling Cheng, Xiaoxiao Sun, and Mingzhong Li. 2020. "High Molecular Weight Silk Fibroin Prepared by Papain Degumming" Polymers 12, no. 9: 2105. https://doi.org/10.3390/polym12092105