Multifunctionality of Polypyrrole Polyethyleneoxide Composites: Concurrent Sensing, Actuation and Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Synthesis of PPy Blends
2.3. Linear Actuation
2.4. Characterization
3. Results and Discussion
3.1. Characterization
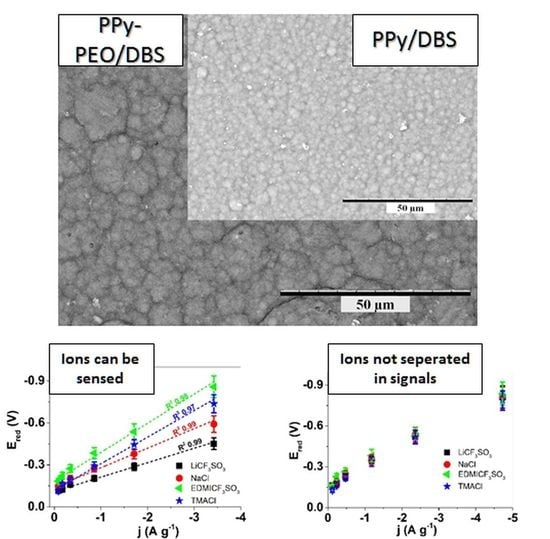
3.1.1. Morphology and Conductivity of Polypyrrole/Dodecylbenzenesulfonate (PPy/DBS) and PPy-PEO (Poly(ethylene Oxide))/DBS Operated in Different Electrolytes
3.1.2. Energy-Dispersive X-ray (EDX) Spectroscopy
3.2. Linear Actuation Controlled by Cyclic Voltammetry
3.3. Energy Storage
3.4. Sensor Calibration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Melling, D.; Martinez, J.G.; Jager, E.W.H. Conjugated Polymer Actuators and Devices: Progress and Opportunities. Adv. Mater. 2019, 31, 1808210. [Google Scholar] [CrossRef] [PubMed]
- Maziz, A.; Concas, A.; Khaldi, A.; Stålhand, J.; Persson, N.-K.; Jager, E.W.H. Knitting and weaving artificial muscles. Sci. Adv. 2017, 3, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutlu, R.; Alici, G.; Li, W. An effective methodology to solve inverse kinematics of electroactive polymer actuators modelled as active and soft robotic structures. Mech. Mach. Theory 2013, 67, 94–110. [Google Scholar] [CrossRef] [Green Version]
- Jager, E.W.H.; Smela, E.; Ingana, O. Microfabricating Conjugated Polymer Actuators. Science 2000, 290, 1540–1545. [Google Scholar] [CrossRef] [Green Version]
- Smela, E. Conjugated polymer actuators for biomedical applications. Adv. Mater. 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Bay, L.; Jacobsen, T.; Skaarup, S.; West, K. Mechanism of actuation in conducting polymers: Osmotic expansion. J. Phys. Chem. B 2001, 105, 8492–8497. [Google Scholar] [CrossRef]
- Otero, T.F. Conducting Polymers: Bioinspired Intelligent Materials and Devices; RSC: Cambridge, UK, 2016; pp. 26–58. [Google Scholar]
- Martinez, J.G.; Otero, T.F.; Jager, E.W.H. Effect of the electrolyte concentration and substrate on conducting polymer actuators. Langmuir 2014, 30, 3894–3904. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Physical and chemical awareness from sensing polymeric artificial muscles. Experiments and modeling. Prog. Polym. Sci. 2015, 44, 62–78. [Google Scholar] [CrossRef]
- Harjo, M.; Zondaka, Z.; Leemets, K.; Järvekülg, M.; Tamm, T.; Kiefer, R. Polypyrrole-coated fiber-scaffolds: Concurrent linear actuation and sensing. J. Appl. Polym. Sci. 2020, 48533, 1–8. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Artificial muscles: A tool to quantify exchanged solvent during biomimetic reactions. Chem. Mater. 2012, 24, 4093–4099. [Google Scholar] [CrossRef]
- Ghosh, S.; Inganäs, O. Networks of Electron-Conducting Polymer in Matrices of Ion-Conducting Polymers Applications to Fast Electrodes. Electrochem. Solid-State Lett. 2000, 3, 213–2015. [Google Scholar] [CrossRef]
- Khadka, R.; Zhang, P.; Nguyen, N.T.; Tamm, T.; Travas-Sejdic, J.; Otero, T.F.; Kiefer, R. Role of polyethylene oxide content in polypyrrole linear actuators. Mater. Today Commun. 2020, 23, 100908. [Google Scholar] [CrossRef]
- Otero, T.F.; Boyano, I. Comparative study of conducting polymers by the ESCR model. J. Phys. Chem. B 2003, 107, 6730–6738. [Google Scholar] [CrossRef]
- Valero, L.; Otero, T.F.; Martinez, J.G.; Martínez, J.G. Exchanged Cations and Water during Reactions in Polypyrrole Macroions from Artificial Muscles. ChemPhysChem 2014, 15, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Harjo, M.; Tamm, T.; Anbarjafari, G.; Kiefer, R. Hardware and Software Development for Isotonic Strain and Isometric Stress Measurements of Linear Ionic Actuators. Polymers 2019, 11, 1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, S.; Rempe, S.B. Coordination numbers of alkali metal ions in aqueous solutions. Biophys. Chem. 2006, 124, 192–199. [Google Scholar] [CrossRef]
- Põldsalu, I.; Rohtlaid, K.; Plesse, C.; Vidal, F.; Nguyen, T.N.; Anna-Liisa, P.; Tarmo, T.; Rudolf, K. Printed PEDOT: PSS Trilayer: Mechanism Evaluation. Materials 2020, 13, 491. [Google Scholar]
- Finney, J.L.; Turner, J. Direct measurement by neutron diffraction of the solvation of polar and apolar molecules: The hydration of the tetramethylammonium ion. Faraday Discuss. Chem. Soc. 1988, 85, 125–135. [Google Scholar] [CrossRef]
- Slusher, J.T.; Cummings, P.T. Molecular simulation study of tetraalkylammonium halides. 1. Solvation structure and hydrogen bonding in aqueous solutions. J. Phys. Chem. B 1997, 101, 3818–3826. [Google Scholar] [CrossRef]
- Khadka, R.; Aydemir, N.; Kesküla, A.; Tamm, T.; Travas-Sejdic, J.; Kiefer, R. Enhancement of polypyrrole linear actuation with poly(ethylene oxide). Synth. Met. 2017, 232, 1–7. [Google Scholar] [CrossRef]
- Kiefer, R.; Khadka, R.; Travas-Sejdic, J. Poly(ethylene oxide) in polypyrrole doped dodecylbenzenesulfonate: Characterisation and linear actuation. Int. J. Nanotechnol. 2018, 15, 689–694. [Google Scholar] [CrossRef]
- Zondaka, Z.; Harjo, M.; Khan, A.; Khanh, T.T.; Tamm, T.; Kiefer, R. Optimal phosphotungstinate concentration for polypyrrole linear actuation and energy storage. Multifunct. Mater. 2018, 1, 14003. [Google Scholar] [CrossRef]
- An, K.H.; Jeon, K.K.; Heo, J.K.; Lim, S.C.; Bae, D.J.; Lee, Y.H. High-Capacitance Supercapacitor Using a Nanocomposite Electrode of Single-Walled Carbon Nanotube and Polypyrrole. J. Electrochem. Soc. 2002, 149, 1058–1062. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, C.Y.; Chang, H.T. Supercapacitors incorporating hollow cobalt sulfide hexagonal nanosheets. J. Power Sources 2011, 196, 7874–7877. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Y.; Han, X.; Xu, S.; Wang, J. Applicable tolerance evaluations of ion-doped carbon nanotube/polypyrrole electrode under adverse solution conditions for capacitive deionization process. Sep. Purif. Technol. 2018, 201, 167–178. [Google Scholar] [CrossRef]
- Sharma, R.K.; Rastogi, A.C.; Desu, S.B. Pulse polymerized polypyrrole electrodes for high energy density electrochemical supercapacitor. Electrochem. Commun. 2008, 10, 268–272. [Google Scholar] [CrossRef]
- García-Córdova, F.; Valero, L.; Ismail, Y.A.; Otero, T.F. Biomimetic polypyrrole based all three-in-one triple layer sensing actuators exchanging cations. J. Mater. Chem. 2011, 21, 17265–17272. [Google Scholar] [CrossRef]
- Otero, T.F.; Cortés, M.T. A sensing muscle. Sens. Actuators B Chem. 2003, 96, 152–156. [Google Scholar] [CrossRef]
- Martinez, J.G.; Otero, T.F. Structural electrochemistry. Chronopotentiometric responses from rising compacted polypyrrole electrodes: Experiments and model. RSC Adv. 2014, 4, 29139. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Z.; Feng, M.; Wang, L.; Wang, Z. Comparative antioxidant status in freshwater fish Carassius auratus exposed to eight imidazolium bromide ionic liquids: A combined experimental and theoretical study. Ecotoxicol. Environ. Saf. 2014, 102, 187–195. [Google Scholar] [CrossRef]
- Mori, I.C.; Arias-Barreiro, C.R.; Koutsaftis, A.; Ogo, A.; Kawano, T.; Yoshizuka, K.; Inayat-Hussain, S.H.; Aoyama, I. Toxicity of tetramethylammonium hydroxide to aquatic organisms and its synergistic action with potassium iodide. Chemosphere 2015, 120, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Electrolyte | PPy-PEO/DBS (S cm−1) | PPy/DBS (S cm−1) |
|---|---|---|
| Direct after Polymerization | 17.7 ± 1.3 | 7.9 ± 0.6 |
| LiCF3SO3 | 9.3 ± 0.4 | 4.8 ± 0.2 |
| NaCl | 8.6 ± 0.6 | 4.6 ± 0.3 |
| EDMICF3SO3 | 6.3 ± 0.3 | 2.8 ± 0.2 |
| TMACl | 9.0 ± 0.5 | 4.4 ± 0.3 |
| Electrolytes | Linear Equation of Electrical Energy Ue (J g−1) = | Linear Equation of Potential at Reduction −Ered (V) = | Strain ε [%]s |
|---|---|---|---|
| LiCF3SO3 | −1.12 ± 0.09 j (A g−1) | 0.1031 ± 0.005 j (A g−1) | 3.1 ± 0.25 j |
| NaCl | −0.91 ± 0.07 j (A g−1) | 0.14 ± 0.008 j (A g−1) | 2.02 ± 0.14 j |
| TMACl | −0.60 ± 0.04 j (A g−1) | 0.19 ± 0.013 j (A g−1) | 2.51 ± 0.17 j |
| EDMICF3SO3 | −1.09 ± 0.08 j (A g−1) | 0.208 ± 0.014 j (A g−1) | 1.55 ± 0.09 j |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuyen, N.Q.; Kiefer, R.; Zondaka, Z.; Anbarjafari, G.; Peikolainen, A.-L.; Otero, T.F.; Tamm, T. Multifunctionality of Polypyrrole Polyethyleneoxide Composites: Concurrent Sensing, Actuation and Energy Storage. Polymers 2020, 12, 2060. https://doi.org/10.3390/polym12092060
Khuyen NQ, Kiefer R, Zondaka Z, Anbarjafari G, Peikolainen A-L, Otero TF, Tamm T. Multifunctionality of Polypyrrole Polyethyleneoxide Composites: Concurrent Sensing, Actuation and Energy Storage. Polymers. 2020; 12(9):2060. https://doi.org/10.3390/polym12092060
Chicago/Turabian StyleKhuyen, Nguyen Quang, Rudolf Kiefer, Zane Zondaka, Gholamreza Anbarjafari, Anna-Liisa Peikolainen, Toribio F. Otero, and Tarmo Tamm. 2020. "Multifunctionality of Polypyrrole Polyethyleneoxide Composites: Concurrent Sensing, Actuation and Energy Storage" Polymers 12, no. 9: 2060. https://doi.org/10.3390/polym12092060