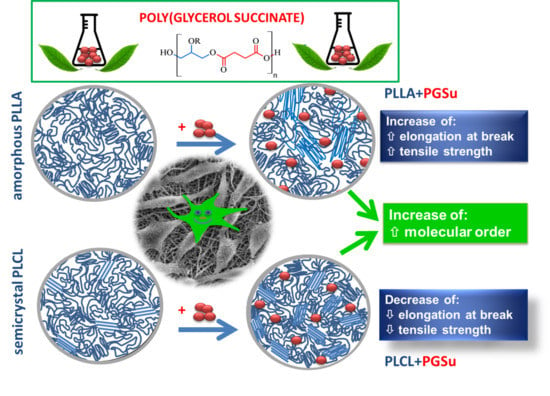
Poly(Glycerol Succinate) as an Eco-Friendly Component of PLLA and PLCL Fibres towards Medical Applications
Abstract
:1. Introduction
2. Experimental
2.1. Oligomer Poly(Glycerol Succinate)
2.1.1. Synthesis
2.1.2. Chemical Structure
2.2. Bicomponent Fibres Fabrication
2.2.1. Materials
2.2.2. Structure and Morphology
2.2.3. Cellular Viability
2.2.4. Statistical Analysis
3. Results
3.1. Oligomer PGSu
3.2. Bicomponent Fibres
3.2.1. Morphology and Structure
3.2.2. Mechanical Properties
3.2.3. Cellular Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howell, B.A.; Lazar, S.T. Biobased Plasticizers from Glycerol/Adipic Acid Hyperbranched Poly(ester)s. Ind. Eng. Chem. Res. 2019, 58, 17227–17234. [Google Scholar] [CrossRef]
- Zhang, T.; Howell, B.A.; Smith, P.B. Rational Synthesis of hyperbranched poly(ester)s. Ind. Eng. Chem. Res. 2017, 56, 1661–1670. [Google Scholar] [CrossRef]
- Khongphow, C.; Theerakul, J.; Puttamat, S.; Singkhonrat, J. Characterisation of poly (glycerol-succinate) oligomers as bio-based non-ionic surfactants by nuclear magnetic resonance and mass spectrometry. Colloids Surf. A Physicochem. Eng. Asp. 2015, 468, 301–308. [Google Scholar] [CrossRef]
- Can, E.; Malta, S.; Kınacı, E.; Çalıkoğlu, A.C.; Köse, G.T. Polybutylene Succinate (PBS)—Polycaprolactone (PCL) Blends Compatibilized with Poly (ethylene oxide)-block-poly (propylene oxide)-block –poly (ethylene oxide) (PEO-PPO-PEO) Copolymer for Biomaterial Applications. Polym.-Plast. Technol. Eng. 2014, 53, 1178–1193. [Google Scholar] [CrossRef]
- Valerio, O.; Misra, M.; Mohanty, A.K. Sustainable biobased blends of poly (lactic acid) (PLA) and poly (glycerol succinate-co-maleate) (PGSMA) with balanced performance prepared by dynamic vulcanization. RSC Adv. 2017, 7, 38594–38603. [Google Scholar] [CrossRef] [Green Version]
- Denis, P.; Wrzecionek, M.; Gadomska-Gajadhur, A.; Sajkiewicz, P. Poly (Glycerol Sebacate)–Poly (l-Lactide) Nonwovens. Towards Attractive Electrospun Material for Tissue Engineering. Polymers 2019, 11, 2113. [Google Scholar] [CrossRef] [Green Version]
- Valerio, O.; Pin, J.M.; Misra, M.; Mohanty, A.K. Synthesis of glycerol-based biopolyesters as toughness enhancers for polylactic acid bioplastic through reactive extrusion. ACS Omega 2016, 1, 1284–1295. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pina, C.D.; Rossi, M.; Pagliaro, M. Understanding the glycerol market. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439. [Google Scholar] [CrossRef]
- Yin, G.Z.; Yang, X.; Zhou, Z.; Li, Q. A Green Pathway to Adjust the Mechanical Properties and Degradation Rate of PCL by Blending Bio-sourced Poly (glycerol-succinate) Oligoesters. Mater. Chem. Front. 2017, 2, 544–553. [Google Scholar] [CrossRef]
- Carnahan, M.A.; Grinstaff, M.W. Synthesis and characterization of poly (glycerol−succinic acid) dendrimers. Macromolecules 2001, 34, 7648–7655. [Google Scholar] [CrossRef]
- Halpern, J.M.; Urbanski, R.; Weinstock, A.K.; Iwig, D.F.; Mathers, R.T.; Von Recum, H.A. A biodegradable thermoset polymer made by esterification of citric acid and glycerol. J. Biomed. Mater. Res. Part A 2014, 102, 1467–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, X.J.; Karim, A.A.; Owh, C. Poly (glycerol sebacate) biomaterial: Synthesis and biomedical applications. J. Mater. Chem. B 2015, 3, 7641–7652. [Google Scholar] [CrossRef] [PubMed]
- Liverani, L.; Piegat, A.; Niemczyk, A.; E Fray, M.; Boccaccini, A.R. Electrospun fibers of poly (butylene succinate–co–dilinoleic succinate) and its blend with poly (glycerol sebacate) for soft tissue engineering applications. Eur. Polym. J. 2016, 81, 295–306. [Google Scholar] [CrossRef]
- Fang, Q.; Hanna, M.A. Rheological properties of amorphous and semicrystalline polylactic acid polymers. Ind. Crop. Prod. 1999, 10, 47–53. [Google Scholar] [CrossRef]
- Sikorska, W.; Adamus, G.; Dobrzynski, P.; Libera, M.; Rychter, P.; Krucińska, I.; Komisarczyk, A.; Cristea, M.; Kowalczuk, M. Forensic engineering of advanced polymeric materials—Part II: The effect of the solvent-free non-woven fabrics formation method on the release rate of lactic and glycolic acids from the tin-free poly (lactide-co-glycolide) nonwovens. Polym. Degrad. Stab. 2014, 110, 518–528. [Google Scholar] [CrossRef]
- Darie-Niţă, R.N.; Vasile, C.; Irimia, A.; Lipşa, R.; Râpă, M. Evaluation of some eco-friendly plasticizers for PLA films processing. J. Appl. Polym. Sci. 2015, 133. [Google Scholar] [CrossRef]
- Gu, L.Q.; Li, Y.F.; Cheng, S.J. Modification of Poly (l-lactide) by Poly (glycerol-sebacate). J. Funct. Polym. 2008, 21, 343–348. [Google Scholar]
- Coativy, G.; Misra, M.; Andrzejewski, J. Microwave Synthesis and Melt Blending of Glycerol Based Toughening Agent with Poly (lactic acid). ACS Sustain. Chem. Eng. 2016, 4, 2142–2149. [Google Scholar] [CrossRef] [Green Version]
- Monnier, X.; Delpouve, N.; Basson, N.; Guinault, A.; Domenek, S.; Saiter, A.; Mallon, P.E.; Dargent, E. Molecular dynamics in electrospun amorphous plasticized polylactidefibers. Polymer 2015, 73, 68–78. [Google Scholar] [CrossRef]
- Perkins, W.G. Polymer toughness and impact resistance. Polym. Eng. Sci. 1999, 39, 2445–2460. [Google Scholar] [CrossRef]
- Dulnik, J.; Kołbuk, D.; Denis, P.; Sajkiewicz, P. The effect of a solvent on cellular response to PCL/gelatin and PCL/collagen electrospun nanofibres. Eur. Polym. J. 2018, 104, 147–156. [Google Scholar] [CrossRef]
- Budnicka, M.; Kołbuk, D.; Ruśkowski, P.; Gadomska-Gajadhur, A. Poly-L-lactide scaffolds with super pores obtained by freeze-extraction method. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stoclet, G.; Seguela, R.; Lefebvre, J.M.; Rochas, C. New Insights on the Strain-Induced Mesophase of Poly (d, l-lactide): In situ WAXS and DSC study of the thermo-mechanical stability. Macromolecules 2010, 43, 7228–7237. [Google Scholar] [CrossRef]
- Ma, Q.; Pyda, M.; Mao, B.; Cebe, P. Relationship between the rigid amorphous phase and mesophase in electrospun fibers. Polymer 2013, 54, 2544–2554. [Google Scholar] [CrossRef]
- Li, J.; Xiao, P.; Li, H.; Zhang, Y.; Xue, F.; Luo, B.; Huang, S.; Shang, Y.; Wen, H.; Christiansen, J.D.C.; et al. Crystalline structures and crystallization behaviors of poly (L-lactide) in poly (L-lactide)/graphene nanosheet composites. Polym. Chem. 2015, 6, 3988–4002. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Howell, B.A.; Dumitrascu, A.; Martin, S.J.; Smith, P.B. Synthesis and characterization of glycerol-adipic acid hyperbranched polyesters. Polymer 2014, 55, 5065–5072. [Google Scholar] [CrossRef]
- Gomez-Sanchez, C.; Kowalczyk, T.; Ruiz De Eguino, G.; Lopez-Arraiza, A.; Infante, A.; Rodriguez, C.I.; Kowalewski, T.A.; Sarrionandia, M.; Aurrekoetxea, J. Electrospinning of poly (lactic acid)/polyhedral oligomeric silsesquioxane nanocomposites and their potential in chondrogenic tissue regeneration. J. Biomater. Sci. Polym. Ed. 2014, 25, 802–825. [Google Scholar] [CrossRef]
- Kołbuk, D.; Sajkiewicz, P.; Maniura-Weber, K.; Fortunato, G. Structure and morphology of electrospun polycaprolactone/gelatine nanofibres. Eur. Polym. J. 2013, 49, 2052–2061. [Google Scholar] [CrossRef]
- Leonés, A.; Sonseca, A.; López, D.; Fiori, S.; Peponi, L. Shape memory effect on electrospun PLA-based fibers tailoring their thermal response. Eur. Polym. J. 2019, 117, 217–226. [Google Scholar] [CrossRef]
- Mangavel, C.; Barbot, J.; Gueguen, J.; Popineau, Y. Molecular Determinants of the influence of hydrophilic plasticizers on the mechanical properties of cast wheat gluten films. J. Agric. Food Chem. 2003, 51, 1447–1452. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Quintard, G.; Massardier-Nageotte, V. Biobased additive plasticizing Polylactic acid (PLA). Polímeros 2015, 25, 581–590. [Google Scholar] [CrossRef]
- Roth, C.B.; Dutcher, J.R. Glass transition and chain mobility in thin polymer films. J. Electroanal. Chem. 2005, 584, 13–22. [Google Scholar] [CrossRef]
- Kołbuk, D.; Sajkiewicz, P.; Denis, P.; Choińska, E. Investigations of polycaprolactone/gelatin blends in terms of their miscibility. Bull. Pol. Acad. Sci. Tech. Sci. 2013, 61, 629–632. [Google Scholar]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering; John Wiley & Sons: New Your, NY, USA, 2011; Volume 5, pp. 344–348. [Google Scholar]
- Kołbuk, D.; Sajkiewicz, P.; Kowalewski, T.A. Optical birefringence and molecular orientation of electrospun polycaprolactone fibers by polarizing-interference microscopy. Eur. Polym. J. 2012, 48, 275–283. [Google Scholar] [CrossRef]
- Murariu, M.; Da Silva Ferreira, A.; Alexandre, M.; Dubois, P. Polylactide (PLA) designed with desired end-use properties: 1. PLA compositions with low molecular weight ester-like plasticizers and related performances. Polym. Adv. Technol. 2008, 19, 636–646. [Google Scholar] [CrossRef]
- Pillin, I.; Montrelay, N.; Grohens, Y. Thermo-mechanical characterization of plasticized PLA: Is the miscibility the only significant factor? Polymer 2006, 47, 4676–4682. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of nucleation and plasticization on the crystallization of poly (lactic acid). Polymer 2007, 48, 6855–6866. [Google Scholar] [CrossRef]
- Kang, H.; Li, Y.; Gong, M.; Guo, Y.; Guo, Z.; Fang, Q.; Li, X. An environmentally sustainable plasticizer toughened polylactide. RSC Adv. 2018, 8, 11643–11651. [Google Scholar] [CrossRef] [Green Version]
- Li, F.J.; Zhang, S.D.; Liang, J.Z.; Wang, J.Z. Effect of polyethylene glycol on the crystallization and impact properties of polylactide-based blends. Polym. Adv. Technol. 2015, 26, 465–475. [Google Scholar] [CrossRef]
- Piorkowska, E.; Kulinski, Z.; Galeski, A.; Masirek, R. Plasticization of semicrystalline poly(l-lactide) with poly(propylene glycol). Polymer 2006, 47, 7178–7188. [Google Scholar] [CrossRef]
- Keith, H.D.; Padden, F.J. Spherulitic Crystallization from the melt. II. Influence of fractionation and impurity segregation on the kinetics of crystallization. J. Appl. Phys. 1964, 35, 1286–1296. [Google Scholar] [CrossRef]
- Keith, H.D.; Padden, F.J.; Vadimsky, R.G. Intercrystalline links in polyethylene crystallized from the melt. J. Polym. Sci. Part A-2 Polym. Phys. 1966, 4, 267–281. [Google Scholar] [CrossRef]
- Böstman, O.; Pihlajamäki, H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: A review. Biomaterials 2000, 21, 2615–2621. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nakiou, E.A.; Christodoulou, E.; Bikiaris, D.N.; Kontonasaki, E.; Liverani, L.; Boccaccini, A.R. Polyglycerol Hyperbranched Polyesters: Synthesis, properties and pharmaceutical and biomedical applications. Int. J. Mol. Sci. 2019, 20, 6210. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Sample Acronym with PLLA | Sample Acronym with PLCL | Main Polymer Amount % (w/w) | PGSu Amount % (w/w) |
|---|---|---|---|
| PLLA0 | PLCL0 | 100 | 0 |
| PLLA2 | PLCL2 | 98 | 5 |
| PLLA5 | PLCL5 | 95 | 5 |
| PLLA10 | PLCL10 | 90 | 10 |
| PLLA20 | PLCL20 | 80 | 20 |
| PLLA40 | PLCL40 | 60 | 40 |
| Sample Name | Tg of PGSu [°C] a | Tg of PLLA [°C] | Enthalpy of Relaxation of PLLA [J/g] | Relaxation Temperature of PLLA [°C] | Cold Crystallisation [J/g] | Tc of PLLA [°C] | Enthalpy of Crystal Phase Melting [J/g] | Tm [°C] | Sample Name | Tg of PGSu [°C] | Enthalpy of Crystal Phase Melting [J/g] | Tm [°C] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLLA0 | b | 50.87 | 15.99 | 55.32 | 13.64 | 67.57 | 45.02 | 180.80 | PLCL0 | - | 24.89 | 157.24 |
| PLLA2 | - | 50.84 | 10.65 | 56.6 | 16.71 | 66.78 | 53.54 | 182.66 | PLCL2 | - | 24.93 | 156.98 |
| PLLA5 | −32.53 | 50.72 | 9.39 | 56.67 | 15.52 | 67.44 | 53.301 | 182.41 | PLCL5 | −15.98 | 25.26 | 157.78 |
| PLLA10 | −32.58 | 50.55 | 8.92 | 56.66 | 15.57 | 67.64 | 51.08 | 181.07 | PLCL10 | −20.56 | 29.66 | 157.04 |
| PLLA20 | −30.92 | 49.21 | 7.86 | 56.97 | 11.36 | 67.89 | 32.58 | 180.18 | PLCL20 | −25.20 | 31.45 | 157.11 |
| PLLA40 | −27.83 | 48.34 | 2.71 | 59.92 | 12.14 | 71.98 | 16.04 | 180.12 | PLCL40 | −30.96 | 27.04 | 156.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolbuk, D.; Jeznach, O.; Wrzecionek, M.; Gadomska-Gajadhur, A. Poly(Glycerol Succinate) as an Eco-Friendly Component of PLLA and PLCL Fibres towards Medical Applications. Polymers 2020, 12, 1731. https://doi.org/10.3390/polym12081731
Kolbuk D, Jeznach O, Wrzecionek M, Gadomska-Gajadhur A. Poly(Glycerol Succinate) as an Eco-Friendly Component of PLLA and PLCL Fibres towards Medical Applications. Polymers. 2020; 12(8):1731. https://doi.org/10.3390/polym12081731
Chicago/Turabian StyleKolbuk, Dorota, Oliwia Jeznach, Michał Wrzecionek, and Agnieszka Gadomska-Gajadhur. 2020. "Poly(Glycerol Succinate) as an Eco-Friendly Component of PLLA and PLCL Fibres towards Medical Applications" Polymers 12, no. 8: 1731. https://doi.org/10.3390/polym12081731