Preparation and Characterization of Semi-Alicyclic Polyimides Containing Trifluoromethyl Groups for Optoelectronic Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Semi-Alicyclic Polyimides and Preparation of Films
3. Results and Discussion
3.1. Synthesis and Characterization of Semi-Alicyclic Polyimides
3.2. Mechanical Properties and Thermal Diffusivities of Semi-alicyclic Polyimides
3.3. Thermal Properties of Semi-Alicyclic Polyimides
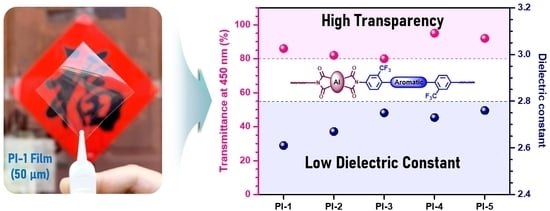
3.4. Refractive and Dielectric Properties of Semi-Alicyclic Polyimides
3.5. Optical Properties of Semi-Alicyclic Polyimides
3.6. Structure–Property Relationships of Semi-Alicyclic Polyimides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loste, J.; Lopez-Cuesta, J.M.; Billon, L.; Garay, H.; Save, M. Transparent polymer nanocomposites: An overview on their synthesis and advanced properties. Prog. Polym. Sci. 2019, 89, 133–158. [Google Scholar] [CrossRef]
- Bae, W.J.; Kovalev, M.K.; Kalinina, F.; Kim, M.; Cho, C. Towards colorless polyimide/silica hybrids for flexible substrates. Polymer 2016, 105, 124–132. [Google Scholar] [CrossRef]
- Kim, S.D.; Lee, B.; Byun, T.; Chung, I.S.; Park, J.; Shin, I.; Ahn, N.Y.; Seo, M.; Lee, Y.; Kim, Y.; et al. Poly(amide-imide) materials for transparent and flexible displays. Sci. Adv. 2018, 4, eaau1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susa, A.; Mordvinkin, A.; Saalwachter, K.; Zwaag, S.V.D.; Garcia, S.J. Identifying the role of primary and secondary interactions on the mechanical properties and healing of densely branched polyimides. Macromolecules 2018, 51, 8333–8345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.Y.; Zhang, Y.; Jiang, G.L.; Zhi, X.X.; Xiao, X.; Wu, L.; Jia, Y.J.; Liu, J.G.; Zhang, X.M. Preparation and properties of inherently black polyimide films with extremely low coefficients of thermal expansion and potential applications for black flexible copper clad laminates. Polymers 2020, 12, 576. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Tsai, C.Y.; Li, L.J.; Liaw, D.J. Colorless-to-colorful switching electrochromic polyimides with very high contrast ratio. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Wang, Z.H.; Fang, G.Q.; He, J.J.; Yang, H.X.; Yang, S.Y. Semi-aromatic thermosetting polyimide resins containing alicyclic units for achieving low melt viscosity and low dielectric constant. React. Funct. Polym. 2020, 146, 104411. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hishiki, T. Poly(ester imide)s possessing low coefficients of thermal expansion and low water absorption (V). Effects of ester-linked diamines with different lengths and substituents. Polymers 2020, 12, 859. [Google Scholar] [CrossRef] [Green Version]
- Fukukawa, K.; Okazaki, M.; Sakata, Y.; Urakami, T.; Yamashita, W.; Tamai, S. Synthesis and properties of multi-block semi-alicyclic polyimides for thermally stable transparent and low CTE film. Polymer 2013, 54, 1053–1063. [Google Scholar] [CrossRef]
- Gong, G.M.; Wu, J.T.; Zhao, Y.; Liu, J.G.; Jin, X.; Jiang, L. A novel fluorinated polyimide surface with petal effect produced by electrospinning. Soft Matter 2014, 10, 549–552. [Google Scholar]
- Hasegawa, M. Development of solution-processable, optically transparent polyimides with ultra-low linear coefficients of thermal expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Guo, C.Y.; Fu, M.G.H.; Zhang, Y.; Yin, L.M.; Wu, L.; Liu, J.G.; Zhang, X.M. Enhancement of solvent resistance of polyimide electrospun mat via the UV-assisted electrospinning and photosensitive varnish. Polymers 2019, 11, 2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ree, M. High performance polyimides for applications in microelectronics and flat panel displays. Macromol. Res. 2006, 14, 1–33. [Google Scholar] [CrossRef]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Tsai, C.L.; Yen, H.J.; Liou, G.S. Highly transparent polyimide hybrids for optoelectronic applications. React. Funct. Polym. 2016, 108, 2–30. [Google Scholar] [CrossRef]
- Takizawa, K.; Fukuchi, S.; Takemasa, C.; Ishige, R.; Asai, S.; Ando, S. Enhancing photoconductivity of aromatic polyimide films by incorporating fluorinated dianhydrides and main chain triphenylamine structure. Polymer 2018, 157, 122–130. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Ha, C.S. Recent trends on transparent colorless polyimides with balanced thermal and optical properties: Design and synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Nam, K.H.; Jin, J.U.; Lee, D.H.; Han, H.; Goh, M.; Yu, J.; Ku, B.C.; You, N.H. Towards solution-processable, thermally robust, transparent polyimide-chain-end tethered organosilicate nanohybrids. Compos. Part B Eng. 2019, 163, 290–296. [Google Scholar] [CrossRef]
- Yu, X.H.; Liu, J.N.; Wu, D.Y. Colorless PI structure design and evaluation for achieving low CTE target. Mater. Today Commun. 2019, 21, 100562. [Google Scholar] [CrossRef]
- Maier, G. Low dielectric constant polymers for microelectronics. Prog. Polym. Sci. 2001, 26, 3–65. [Google Scholar] [CrossRef]
- Li, X.; Lei, H.; Guo, J.; Wang, J.; Qi, S.; Tian, G.; Wu, D. Composition design and properties investigation of BPDA/PDA/TFDB co-polyimide films with low dielectric permittivity. J. Appl. Polym. Sci. 2019, 136, 47989. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, G.; Zhou, Y.; Li, X.; Fang, X. Synthesis and characterization of organosoluble and transparent polyimides derived from trans-1,2-bis(3,4-dicarboxyphenoxy)cyclohexane dianhydride. J. Appl. Polym. Sci. 2015, 132, 42317. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ozawa, H.; Ishiguro, E.; Komatsu, S. Properties of alicyclic polyimides with bis-spironorbornane structure prepared in various solvents. J. Photopolym. Sci. Tec. 2016, 29, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Liu, J.; Liu, X.; Wang, Y.; Gao, X.; Meng, X.; Tu, G.L. High performance soluble polyimides from ladder-type fluorinated dianhydride with polymorphism. Polymers 2018, 10, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanosue, K.; Hirata, S.; Vacha, M.; Augulis, R.; Gulbinas, V.; Ishige, R.; Ando, S. A colorless semi-aromatic polyimide derived from a sterically hindered bromine-substituted dianhydride exhibiting dual fluorescence and phosphorescence emission. Mater. Chem. Front. 2019, 3, 39–49. [Google Scholar] [CrossRef]
- Liu, H.; Zhai, L.; Bai, L.; He, M.; Wang, C.; Mo, S.; Fan, L. Synthesis and characterization of optically transparent semi-aromatic polyimide films with low fluorine content. Polymer 2019, 163, 106–114. [Google Scholar] [CrossRef]
- Hasegawa, M.; Koyanaka, M. Polyimides containing trans-1,4-cyclohexane unit. Polymerizability of their precursors and low-CTE and low-K and high-Tg properties. High Perform. Polym. 2003, 15, 47–64. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horiuchi, M.; Kumakura, K.; Koyama, J. Colorless polyimides with low coefficient of thermal expansion derived from alkyl-substituted cyclobutanetetracarboxylic dianhydrides. Polym. Int. 2014, 63, 486–500. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Gao, Z.; Yang, H.; Yang, S. Synthesis and properties of fluorinated semi–alicyclic transparent polyimide films for optocommunication applications. Chin. J. Mater. Res. 2008, 22, 615–618. [Google Scholar]
- Chen, Z.; Zhou, Y.; Wu, Y.; Liu, S.; Huang, H.; Zhao, J. Fluorinated polyimide with polyhedral oligomeric silsesquioxane aggregates: Toward low dielectric constant and high toughness. Compos. Sci. Technol. 2019, 181, 107700. [Google Scholar] [CrossRef]
- Qian, C.; Bei, R.; Zhu, T.; Zheng, W.; Liu, S.; Chi, Z.; Aldred, M.P.; Chen, X.; Zhang, Y.; Xu, J. Facile strategy for intrinsic low-k dielectric polymers: Molecular design based on secondary relaxation behavior. Macromolecules 2019, 52, 4601–4609. [Google Scholar] [CrossRef]
- Qiu, G.; Ma, W.; Jiao, Y.; Wu, L. Low-dielectric-constant aromatic homopolyimide and copolyimide derived from pyromellitic dianhydride, 4,4’-oxydianiline,2,2-bis[4-(4-aminephenoxy)phenyl]propane, 1,4-bis(4-aminophenoxy)benzene, or 1,3-bis(4-amino- phenoxy)benzene. J. Appl. Polym. Sci. 2019, 136, 47405. [Google Scholar] [CrossRef]
- Susa, A.; Bijleveld, J.; Santana, M.H.; Garcia, S.J. Understanding the effect of the dianhydride structure on the properties of semiaromatic polyimides containing a biobased fatty diamine. ACS Sustain. Chem. Eng. 2018, 6, 668–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, Y.B.; Seong, J.G.; Lee, Y.M. Polyimides containing aliphatic/alicyclic segments in the main chains. Prog. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, X.; Zheng, F.; Lu, X.; Lu, Q. Synthesis of highly transparent and heat-resistant polyimides containing bulky pendant moieties. Polym. Int. 2019, 68, 1186–1193. [Google Scholar] [CrossRef]
- Yang, C.P.; Su, Y.Y.; Wu, K.L. Organosoluble and light-colored fluorinated polyimides from 2,2-bis[4-(4-amino-2-trifluoromethylphenoxy)phenyl]sulfone and aromatic dianhydrides. J. Polym. Res. 2005, 12, 257–269. [Google Scholar] [CrossRef]
- Zhai, L.; Yang, S.Y.; Fan, L. Preparation and characterization of highly transparent and colorless semi-aromatic polyimide films derived from alicyclic dianhydride and aromatic diamines. Polymer 2012, 53, 3529–3539. [Google Scholar] [CrossRef]
- Hu, X.; Yan, J.; Wang, Y.; Mu, H.; Wang, Z.; Cheng, H.; Zhao, F.; Wang, Z. Colorless polyimides derived from 2R,5R,7S,10S-naphthanetetracarboxylic dianhydride. Polym. Chem. 2017, 8, 6165–6172. [Google Scholar] [CrossRef]
- Zhang, R.; Li, T.Y.; Zhou, H.B.; Huang, H.H.; Chen, Y.M. Biobased transparent polyimides with excellent solubility and mechanical properties using myo-inositol derived diamines. React. Funct. Polym. 2018, 128, 91–96. [Google Scholar] [CrossRef]
- Yu, H.C.; Jung, J.W.; Choi, J.Y.; Chung, C.M. Kinetic study of low-temperature imidization of poly(amic acid)s and preparation of colorless, transparent polyimide films. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1593–1602. [Google Scholar] [CrossRef]
- Yorifuji, D.; Ando, S. Molecular structure dependence of out-of-plane thermal diffusivities in polyimide films: A key parameter for estimating thermal conductivity of polymers. Macromolecules 2010, 43, 7583–7593. [Google Scholar] [CrossRef]
- Zhuang, Y.B.; Ando, S. Evaluation of free volume and anisotropic chain orientation of Troger’s base (TB)-based microporous polyimide/copolyimide membranes. Polymer 2017, 123, 39–48. [Google Scholar] [CrossRef]
- Xie, K.; Zhang, S.Y.; Liu, J.G.; He, M.H.; Yang, S.Y. Synthesis and characterization of soluble fluorine-containing polyimides based on 1,4-bis(4-amino-2-trifluoromethylphenoxy)benzene. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2581–2590. [Google Scholar] [CrossRef]
- Bai, L.; Zhai, L.; He, M.; Wang, C.; Mo, S.; Fan, L. Effect of high temperature annealing on thermal expansion behavior of poly(amide-imide) films with ultralow coefficient of thermal expansion. Acta Polym. Sin. 2019, 50, 1305–1313. [Google Scholar]
- Hasegawa, M.; Tokunaga, R.; Hashimoto, K.; Ishii, J. Crosslinkable polyimides obtained from a reactive diamine and the effect of crosslinking on the thermal properties. React. Funct. Polym. 2019, 139, 181–188. [Google Scholar] [CrossRef]
- Ree, M.; Shin, T.J.; Lee, S.W. Fully rod-like aromatic polyimides: Structure, properties, and chemical modifications. Korea Polym. J. 2001, 9, 1–19. [Google Scholar]
- Ishige, R.; Tanaka, K.; Ando, S. In situ analysis of chain orientation behavior in thin film aromatic polyimides by variable temperature pMAIRS during thermal imidization. Macromol. Chem. Phys. 2018, 219, 1700370. [Google Scholar] [CrossRef]
- Bai, L.; Zhai, L.; He, M.; Wang, C.; Mo, S.; Fan, L. Preparation of heat-resistant poly (amide-imide) films with ultralow coefficients of thermal expansion for optoelectronic application. React. Funct. Polym. 2019, 141, 155–164. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, C.; Guo, H.; Fan, L.; Yang, S.; Sun, W.H. Structure effect on transition mechanism of UV–visible absorption spectrum in polyimides: A density functional theory study. Polymer 2018, 148, 356–369. [Google Scholar] [CrossRef]
- Xu, W.; Ma, X.; Su, Y.; Song, Y.; Shang, M.; Lu, X.; Lu, Q. Synthesis of highly transparent and thermally stable copolyimide with fluorine-containing dianhydride and alicyclic dianhydride. J. Appl. Polym. Sci. 2020, 137, 48603. [Google Scholar] [CrossRef]







| PIs | Molecular Weight | Fluorine Content (%) | WA (%) | CA (°) | ||
|---|---|---|---|---|---|---|
| Mn × 104(g/mol) | Mw × 104 (g/mol) | Mw/Mn | ||||
| PI-1 | 5.84 | 8.35 | 1.43 | 21.4 | 0.78 | 93.7 |
| PI-2 | 5.86 | 8.32 | 1.42 | 17.8 | 0.92 | 92.8 |
| PI-3 | 5.08 | 7.54 | 1.48 | 15.9 | 1.08 | 89.7 |
| PI-4 | 6.77 | 9.23 | 1.36 | 14.6 | 0.77 | 89.2 |
| PI-5 | 6.91 | 8.76 | 1.27 | 15.6 | 1.05 | 89.4 |
| PIs | TS (MPa) | TM (GPa) | EB (%) | D⊥ (×10−8 m2/s) |
|---|---|---|---|---|
| PI-1 | 97.9 ± 2.1 | 2.7 ± 0.1 | 5.1 ± 0.4 | 4.7 |
| PI-2 | 84.4 ± 3.5 | 2.1 ± 0.2 | 8.0 ± 0.4 | 6.0 |
| PI-3 | 77.1 ± 2.8 | 2.0 ± 0.1 | 10.1 ± 0.4 | 5.0 |
| PI-4 | 94.5 ± 2.0 | 2.3 ± 0.1 | 9.1 ± 0.6 | 5.9 |
| PI-5 | 90.6 ± 3.0 | 2.9 ± 0.1 | 8.0 ± 0.5 | 5.0 |
| PIs | Tg (°C) | Tonset (°C) | T5% (°C) | T10% (°C) | R700 (%) | CTE (ppm/°C) |
|---|---|---|---|---|---|---|
| PI-1 | 390 | 422 | 412 | 421 | 16.4 | 34 |
| PI-2 | 285 | 411 | 405 | 412 | 18.5 | 62 |
| PI-3 | 304 | 416 | 410 | 419 | 24.6 | 55 |
| PI-4 | 294 | 418 | 405 | 417 | 28.1 | 63 |
| PI-5 | 295 | 436 | 440 | 455 | 50.0 | 64 |
| PIs | n∥ | n⊥ | nav | ∆n | εopt | ∆ε |
|---|---|---|---|---|---|---|
| PI-1 | 1.5491 | 1.5273 | 1.5418 | 0.0218 | 2.61 | 0.0738 |
| PI-2 | 1.5641 | 1.5486 | 1.5589 | 0.0155 | 2.67 | 0.0531 |
| PI-3 | 1.5909 | 1.5615 | 1.5811 | 0.0294 | 2.75 | 0.1019 |
| PI-4 | 1.5829 | 1.5640 | 1.5766 | 0.0189 | 2.73 | 0.0654 |
| PI-5 | 1.5916 | 1.5669 | 1.5833 | 0.0247 | 2.76 | 0.0858 |
| Kapton | 1.7270 | 1.6785 | 1.7108 | 0.0485 | 3.22 | 0.1817 |
| PIs | Transmittance a | Color Parameters b | |||||
|---|---|---|---|---|---|---|---|
| λ0 (nm) | T400 (%) | T450 (%) | T500 (%) | L* | a* | b* | |
| PI-1 | 298 | 83 | 86 | 93 | 89.4 | −0.2 | 3.8 |
| PI-2 | 303 | 74 | 82 | 88 | 91.0 | −0.1 | 4.0 |
| PI-3 | 313 | 68 | 80 | 85 | 91.7 | −0.3 | 4.2 |
| PI-4 | 299 | 87 | 95 | 98 | 92.3 | −0.2 | 3.4 |
| PI-5 | 301 | 84 | 92 | 95 | 91.2 | −0.1 | 4.0 |
| Kapton | 444 | 0 | 2 | 58 | 79.6 | 6.3 | 107.7 |
| Model Compounds | 2θ (deg) | d (Å) | ELUMO (eV) | EHOMO (eV) | EGAP (eV) |
|---|---|---|---|---|---|
| BODA/TFMB (PI-1) | 15.3 | 5.7 | −1.55 | −6.98 | 5.43 |
| BODA/6FAPB (PI-2) | 16.9 | 5.2 | −1.42 | −6.63 | 5.21 |
| BODA/6FBAB (PI-3) | 17.4 | 5.0 | −1.49 | −6.41 | 4.92 |
| BODA/6FBAS (PI-4) | 16.5 | 5.4 | −1.77 | −6.83 | 5.06 |
| CBDA/6FBAS (PI-5) | 17.8 | 5.0 | −1.89 | −6.87 | 4.98 |
| PMDA/ODA (Kapton) | 18.1 | 4.9 | −3.42 | −6.30 | 2.88 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Liu, W.; Gao, X.; Cui, P.; Zou, T.; Hu, G.; Tao, L.; Zhai, L. Preparation and Characterization of Semi-Alicyclic Polyimides Containing Trifluoromethyl Groups for Optoelectronic Application. Polymers 2020, 12, 1532. https://doi.org/10.3390/polym12071532
Zhang M, Liu W, Gao X, Cui P, Zou T, Hu G, Tao L, Zhai L. Preparation and Characterization of Semi-Alicyclic Polyimides Containing Trifluoromethyl Groups for Optoelectronic Application. Polymers. 2020; 12(7):1532. https://doi.org/10.3390/polym12071532
Chicago/Turabian StyleZhang, Mei, Weili Liu, Xia Gao, Peng Cui, Tao Zou, Guanghui Hu, Liming Tao, and Lei Zhai. 2020. "Preparation and Characterization of Semi-Alicyclic Polyimides Containing Trifluoromethyl Groups for Optoelectronic Application" Polymers 12, no. 7: 1532. https://doi.org/10.3390/polym12071532