Less Reactive Thiol Ligands: Key towards Highly Mucoadhesive Drug Delivery Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of S-Protected Thiol Ligands
2.2.1. Synthesis of Cys-MNA
2.2.2. Purification of Cys-MNA
2.2.3. Synthesis of Cys-NAC
2.2.4. Purification of Cys-NAC
2.2.5. Liquid Chromatography–Mass Spectrometry (LC–MS)
2.3. Synthesis of Polyacrylic Acid Anhydride
2.4. Synthesis of s-Protected Thiolated PAA Conjugates
2.4.1. Synthesis of PAA-Cys-MNA
2.4.2. Synthesis of PAA-Cys-NAC
2.5. FT-IR and 1H-NMR Spectroscopy
2.6. Chemical Characterization of Thiolated PAA Conjugates
2.7. Cytotoxicity Studies on Caco-2 Cell Line
2.8. In-vitro Rheological Investigations
2.9. Swelling Behavior
2.10. In-vitro Mucoadhesion Studies
2.10.1. Tensile Studies
2.10.2. Mucoadhesion Using Flow-Through Method
Fluorescent Labeling of Unmodified and s-Protected Thiolated PAA
Mucoadhesion Study
2.11. Mucus Diffusion Study
2.12. Statistical Analysis
3. Results and discussion
3.1. Synthesis and Characterization of S-Protected Thiolated PAA Conjugates
3.2. Cytotoxicity Studies
3.3. Rheological Investigations
3.4. Swelling Behavior
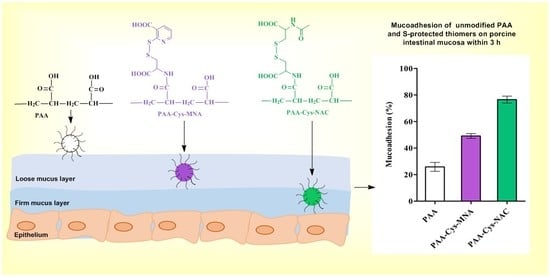
3.5. In-Vitro Mucoadhesion Studies
3.6. Mucus Diffusion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ishida, M.; Nambu, N.; Nagai, T. Highly viscous gel ointment containing Carbopol for application to the oral mucosa. Chem. Pharm. Bull. 1983, 31, 4561–4564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, T. Adhesive topical drug delivery system. J. Control. Release 1985, 2, 121–134. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2010, 11, 748–764. [Google Scholar] [CrossRef]
- Valenta, C. The use of mucoadhesive polymers in vaginal delivery. Adv. Drug Deliv. Rev. 2005, 57, 1692–1712. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Schwarz, V.; Steininger, S. Polymers with thiol groups: a new generation of mucoadhesive polymers? Pharm. Res. 1999, 16, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sinha, V.R. Thiomer: A potential carrier for therapeutic delivery. React. Funct. Polym. 2013, 73, 1156–1166. [Google Scholar] [CrossRef]
- Duggan, S.; Cummins, W.; Donovan, O.O.; Hughes, H.; Owens, E. Thiolated polymers as mucoadhesive drug delivery systems. Eur. J. Pharm. Sci. 2017, 100, 64–78. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef]
- Iqbal, J.; Shahnaz, G.; Dünnhaupt, S.; Müller, C.; Hintzen, F.; Bernkop-Schnürch, A. Preactivated thiomers as mucoadhesive polymers for drug delivery. Biomaterials 2011, 33, 1528–1535. [Google Scholar] [CrossRef] [Green Version]
- Dünnhaupt, S.; Barthelmes, J.; Rahmat, D.; Leithner, K.; Thurner, C.C.; Friedl, H.; Bernkop-Schnürch, A. S-Protected Thiolated Chitosan for Oral Delivery of Hydrophilic Macromolecules: Evaluation of Permeation Enhancing and Efflux Pump Inhibitory Properties. Mol. Pharm. 2012, 9, 1331–1341. [Google Scholar] [CrossRef]
- Netsomboon, K.; Partenhauser, A.; Rohrer, J.; Sündermann, N.E.; Prüfert, F.; Suchaoin, W.; Laffleur, F.; Bernkop-Schnürch, A. Preactivated thiomers for intranasal delivery of apomorphine: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 109, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.; Lopalco, A.; Lopedota, A.; Cutrignelli, A.; Laquintana, V.; Franco, M.; Bernkop-Schnürch, A.; Denora, N. S-preactivated thiolated glycol chitosan useful to combine mucoadhesion and drug delivery. Eur. J. Pharm. Biopharm. 2018, 132, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Bernkop-Schnürch, A. Preactivated thiomers: their role in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 1269–1281. [Google Scholar] [CrossRef]
- Lehr, C.; Poelma, F.G.; Junginger, H.E.; Tukker, J.J. An estimate of turnover time of intestinal mucus gel layer in the rat in situ loop. Int. J. Pharm. 1991, 70, 235–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, S.; Deng, F.; Chen, X.; Wang, X.; Wang, Y.; Zhang, H.; Dai, W.; He, B.; Zhang, Q.; et al. The function and mechanism of preactivated thiomers in triggering epithelial tight junctions opening. Eur. J. Pharm. Biopharm. 2018, 133, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Leichner, C.; Jelkmann, M.; Bernkop-Schnürch, A. Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature. Adv. Drug Deliv. Rev. 2019, 152, 191–221. [Google Scholar] [CrossRef] [PubMed]
- Baus, R.A.; Innerhofer, J.; Rohrer, J.; Lupo, N.; Bernkop-Schnurch, A. Anhydrous thiomers: Strategy for enhanced mucoadhesion. Eur. J. Pharm. Biopharm. 2018, 129, 273–281. [Google Scholar] [CrossRef]
- Hintzen, F.; Hauptstein, S.; Perera, G.; Bernkop-Schnürch, A. Synthesis and in vitro characterization of entirely s-protected thiolated pectin for drug delivery. Eur. J. Pharm. Biopharm. 2013, 85, 1266–1273. [Google Scholar] [CrossRef]
- Shahzadi, I.; Asim, M.H.; Dizdarević, A.; Wolf, J.D.; Kurpiers, M.; Matuszczak, B.; Bernkop-Schnürch, A. Arginine-based cationic surfactants: Biodegradable auxiliary agents for the formation of hydrophobic ion pairs with hydrophilic macromolecular drugs. J. Colloid Interface Sci. 2019, 552, 287–294. [Google Scholar] [CrossRef]
- Mizrahi, B.; Domb, A.J. Anhydride Prodrug of Ibuprofen and Acrylic Polymers. AAPS PharmSciTech 2009, 10, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Simpson, R. Estimation of Free Thiols and Disulfide Bonds Using Ellman’s Reagent. Cold Spring Harb. Protoc. 2008, 2008. [Google Scholar] [CrossRef] [PubMed]
- Fürst, A.; Baus, R.A.; Lupo, N.; Bernkop-Schnürch, A. Entirely S-Protected Thiolated Silicone: A Novel Hydrophobic Mucoadhesive and Skin Adhesive. J. Pharm. Sci. 2019, 108, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, T.; De Cerain, A.L.; Irache, J.M.; Martín-Arbella, N.; Wilcox, M.; Pearson, J.; Azqueta, A. Evaluation of the cytotoxicity, genotoxicity and mucus permeation capacity of several surface modified poly(anhydride) nanoparticles designed for oral drug delivery. Int. J. Pharm. 2017, 517, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Leder, N.; Barthelmes, J. In vitro evaluation of thio-poly acrylic acid for intraoral delivery. Drug Deliv. 2015, 23, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Imam, M.E. Evidence for the interpenetration of mucoadhesive polymers into the mucus gel layer. S.T.P. Pharma Sci. 2003, 13, 171–176. [Google Scholar]
- Asim, M.H.; Nazir, I.; Jalil, A.; Matuszczak, B.; Bernkop-Schnürch, A. Tetradeca-thiolated cyclodextrins: Highly mucoadhesive and in-situ gelling oligomers with prolonged mucosal adhesion. Int. J. Pharm. 2020, 577, 119040. [Google Scholar] [CrossRef]
- Akkus, Z.B.; Nazir, I.; Jalil, A.; Tribus, M.; Bernkop-Schnürch, A. Zeta Potential Changing Polyphosphate Nanoparticles: A Promising Approach To Overcome the Mucus and Epithelial Barrier. Mol. Pharm. 2019, 16, 2817–2825. [Google Scholar] [CrossRef]
- Kriwet, B. Interactions between bioadhesive poly(acrylic acid) and calciumions. Int. J. Pharm. 1996, 127, 135–145. [Google Scholar] [CrossRef]
- Netsomboon, K.; Jalil, A.; Laffleur, F.; Hupfauf, A.; Gust, R.; Bernkop-Schnürch, A. Thiolated chitosans: Are Cys-Cys ligands key to the next generation? Carbohydr. Polym. 2020, 116395. [Google Scholar] [CrossRef]
- Marschütz, M.K.; Bernkop-Schnürch, A. Thiolated polymers: self-crosslinking properties of thiolated 450 kDa poly(acrylic acid) and their influence on mucoadhesion. Eur. J. Pharm. Sci. 2002, 15, 387–394. [Google Scholar] [CrossRef]
- Jalil, A.; Asim, M.H.; Le, N.-M.N.; Laffleur, F.; Matuszczak, B.; Tribus, M.; Bernkop–Schnürch, A. s-protected gellan gum: Decisive approach towards mucoadhesive antimicrobial vaginal films. Int. J. Boil. Macromol. 2019, 130, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.T.; Leonaviciute, G.; Zupančič, O.; Bernkop-Schnürch, A. Thiomers: Impact of in situ cross-linkers on mucoadhesive properties. Eur. J. Pharm. Sci. 2017, 106, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Leitner, V.M.; Walker, G.F.; Bernkop-Schnürch, A. Thiolated polymers: evidence for the formation of disulphide bonds with mucus glycoproteins. Eur. J. Pharm. Biopharm. 2003, 56, 207–214. [Google Scholar] [CrossRef]












| Product | Free Thiols (µmol/g) | Thiols after Reduction with NaBH4 (µmol/g) |
|---|---|---|
| PAA-Cys-MNA | 43.22 ± 21 | 660.65 ± 42.7 |
| PAA-Cys-NAC | 20.84 ± 15 | 683.50 ± 27.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzadi, I.; Fürst, A.; Akkus-Dagdeviren, Z.B.; Arshad, S.; Kurpiers, M.; Matuszczak, B.; Bernkop-Schnürch, A. Less Reactive Thiol Ligands: Key towards Highly Mucoadhesive Drug Delivery Systems. Polymers 2020, 12, 1259. https://doi.org/10.3390/polym12061259
Shahzadi I, Fürst A, Akkus-Dagdeviren ZB, Arshad S, Kurpiers M, Matuszczak B, Bernkop-Schnürch A. Less Reactive Thiol Ligands: Key towards Highly Mucoadhesive Drug Delivery Systems. Polymers. 2020; 12(6):1259. https://doi.org/10.3390/polym12061259
Chicago/Turabian StyleShahzadi, Iram, Andrea Fürst, Zeynep Burcu Akkus-Dagdeviren, Shumaila Arshad, Markus Kurpiers, Barbara Matuszczak, and Andreas Bernkop-Schnürch. 2020. "Less Reactive Thiol Ligands: Key towards Highly Mucoadhesive Drug Delivery Systems" Polymers 12, no. 6: 1259. https://doi.org/10.3390/polym12061259