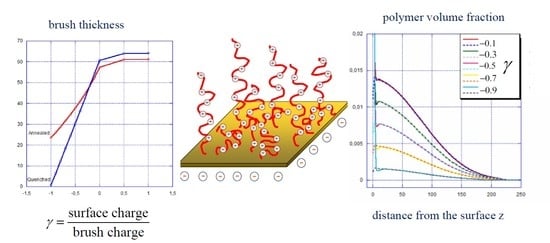
Electroresponsive Polyelectrolyte Brushes Studied by Self-Consistent Field Theory
Abstract
:1. Introduction
2. Model
3. Analytical Results for Polyelectrolyte Brushes Grafted to a Neutral Surface
3.1. Strong Polyelectrolyte Brush, α = const
3.2. Weak (pH-Sensitive) Polyelectrolyte Brush
4. Polyelectrolyte Brush Under Applied Electrical Field: Numerical SF-SCF Results
4.1. Strong Polyelectrolyte Brush, α = const
4.2. Weak vs. Strong Polyelectrolyte Brush
4.3. Relationship Between Surface Charge and Surface Potential
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, W.-L.; Cordero, R.; Tran, H.; Ober, C.K. 50th Anniversary Perspective: Polymer Brushes: Novel Surfaces for Future Materials. Macromolecules 2017, 50, 4089–4113. [Google Scholar] [CrossRef]
- Willott, J.D.; Murdoch, T.J.; Webber, G.B.; Wanless, E.J. Physicochemical behaviour of cationic polyelectrolyte brushes. Prog. Polym. Sci. 2017, 64, 52–75. [Google Scholar] [CrossRef]
- Toomey, R.; Tirrell, M. Functional Polymer Brushes in Aqueous Media from Self-Assembled and Surface-Initiated Polymers. Ann. Rev. Phys. Chem. 2008, 59, 493–517. [Google Scholar] [CrossRef] [PubMed]
- Raviv, U.; Giasson, S.; Kampf, N.; Gohy, J.-F.; Jerome, R.; Klein, J. Lubrication by charged polymers. Nature 2003, 425, 163–165. [Google Scholar] [CrossRef]
- Ruhe, J.; Ballauff, M.; Biesalski, M.; Dziezok, P.; Gruhn, F.; Johannsmann, D.; Houbenov, N.; Hugenberg, N.; Konradi, R.; Minko, S.; et al. Polyelectrolyte Brushes. Adv. Polym. Sci. 2004, 165, 79–150. [Google Scholar]
- Pincus, P.A. Colloid stabilization with grafted polyelectrolytes. Macromolecules 1991, 24, 2912–2919. [Google Scholar] [CrossRef]
- Borisov, O.V.; Birshtein, T.M.; Zhulina, E.B. Collapse of Grafted Polyelectrolyte Layer. J. Phys. II 1991, 1, 521–526. [Google Scholar] [CrossRef]
- Ross, R.; Pincus, P. The polyelectrolyte brush: Poor solvent. Macromolecules 1992, 25, 2177–2183. [Google Scholar] [CrossRef]
- Wittmer, J.; Joanny, J.-F. Charged diblock copolymers at interfaces. Macromolecules 1993, 26, 2691–2697. [Google Scholar] [CrossRef]
- Borisov, O.V.; Zhulina, E.B.; Birshtein, T.M. Diagram of the States of a Grafted Polyelectrolyte Layer. Macromolecules 1994, 27, 4795–4803. [Google Scholar] [CrossRef]
- Miklavic, S.J.; Marcelja, S. Interaction of surfaces carrying grafted polyelectrolytes. J. Phys. Chem. 1988, 92, 6718–6722. [Google Scholar] [CrossRef]
- Misra, S.; Varanasi, S.; Varanasi, P.P. A polyelectrolyte brush theory. Macromolecules 1989, 22, 4173–4179. [Google Scholar] [CrossRef]
- Israels, R.; Leermakers, F.A.M.; Fleer, G.J.; Zhulina, E.B. Charged Polymeric Brushes: Structure and Scaling Relations. Macromolecules 1994, 27, 3249–3261. [Google Scholar] [CrossRef]
- Israels, R.; Leermakers, F.A.M.; Fleer, G.J. On the Theory of Grafted Weak Polyacids. Macromolecules 1994, 27, 3087–3093. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V. Structure and Interactions of Weakly Charged Polyelectrolyte Brushes: Self-Consistent Field Theory. J. Chem. Phys. 1997, 107, 5952–5967. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Klein Wolterink, J.; Borisov, O.V. Screening Effects in Polyelectrolyte Brush: Self-Consistent Field Theory. Macromolecules 2000, 33, 4945–4953. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V. Poisson-Boltzmann Theory of pH-Sensitive (Annealing) Polyelectrolyte Brush. Langmuir 2011, 27, 10615–10633. [Google Scholar] [CrossRef]
- Matsen, M.W. Compression of polyelectrolyte brushes in a salt-free theta solvent. Eur. Phys. J. E 2011, 34, 45–57. [Google Scholar] [CrossRef]
- Ballauff, M.; Borisov, O.V. Polyelectrolyte brushes. Curr. Opin. Colloid Interface Sci. 2006, 11, 316–323. [Google Scholar] [CrossRef]
- Ulasevich, S.A.; Brezesinski, G.; Möhwald, H.; Fratzl, P.; Schacher, F.H.; Poznyak, S.K.; Andreeva, D.V.; Skorb, E.V. Light-Induced Water Splitting Causes High-Amplitude Oscillation of pH-Sensitive Layer-by-Layer Assemblies on TiO2. Angew. Chem. Int. Ed. 2016, 55, 13001–13004. [Google Scholar] [CrossRef]
- Wendler, F.; Tom, J.C.; Schacher, F.H. Synthesis and self-assembly of photoacid-containing block copolymers cased on 1-naphthol. Polym. Chem. 2019, 10, 5602–5616. [Google Scholar] [CrossRef]
- Wendler, F.; Sitting, M.; Tom, J.C.; Dietzek, B.; Schacher, F.H. Polymeric Photoacids based on Naphthols -Design Criteria, Photostability, and Light-Mediated Release. Chem. A Eur. J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.P.; Heriot, S.Y.; Martin, S.J.; Parnell, A.J.; Holt, S.A.; Webster, J.R.P.; Jones, R.A.L. Voltage-Induced Swelling and Deswelling of Weak Polybase Brushes. Langmuir 2011, 27, 11000–11007. [Google Scholar] [CrossRef]
- Drummond, C. Electric-Field-Induced Friction Reduction and Control. Phys. Rev. Lett. 2012, 109, 154302. [Google Scholar] [CrossRef] [PubMed]
- Borisova, O.V.; Billon, L.; Richter, R.P.; Reimhult, E.; Borisov, O.V. pH- and Electro-Responsive Properties of Poly(acrylic acid) and Poly(acrylic acid)-block-poly(acrylic acid-grad-styrene) Brushes Studied by Quartz Crystal Microbalance with Dissipation Monitoring. Langmuir 2015, 31, 7684–7694. [Google Scholar] [CrossRef] [PubMed]
- Senechal, V.; Saadaoui, H.; Rodriguez-Hernandenz, J.; Drummond, C. Electro-responsive polyelectrolyte-coated surfaces. Faraday Discuss. 2017, 199, 335–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senechal, V.; Saadaoui, H.; Rodriguez-Hernandenz, J.; Drummond, C. Electrowetting of Weak Polyelectrolyte-Coated Surface. Langmuir 2017, 33, 4996–5005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borisov, O.V.; Leermakers, F.A.M.; Fleer, G.J.; Zhulina, E.B. Polyelectrolytes Tethered to a Similarly Charged Surface. J. Chem. Phys. 2001, 114, 7700–7712. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V.; van Male, J.; Leermakers, F.A.M. Adsorption of Tethered Polyelectrolytes onto Oppositely Charged Solid-Liquid Interfaces. Langmuir 2001, 17, 1277–1293. [Google Scholar] [CrossRef]
- Yamamoto, T.; Pincus, P.A. Collapse of polyelectrolyte brushes in electric fields. Europhys. Lett. 2011, 95, 48003. [Google Scholar] [CrossRef]
- Tong, C. Numerical self-consistent field theory study of the response of strong polyelectrolyte brushes to external electrical fields. J. Chem. Phys. 2015, 143, 054903. [Google Scholar] [CrossRef] [PubMed]
- Merlitz, H.; Li, C.; Wua, C.; Sommer, J.-U. Polyelectrolyte brushes in External fields: molecular dynamics simulations and mean-field theory. Soft Matter 2015, 11, 5688–5696. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.N. Contribution to the Theory of Microphase Layering in Block-Copolymer Melts. Sov. Phys. JETP 1985, 61, 733–742. [Google Scholar]
- Fleer, G.J.; Cohen Stuart, M.A.; Scheutjens, J.M.H.M.; Cosgrove, T.; Vincent, B. Polymers at Interfaces; Chapman & Hall: London, UK, 1993. [Google Scholar]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okrugin, B.M.; Richter, R.P.; Leermakers, F.A.M.; Neelov, I.M.; Zhulina, E.B.; Borisov, O.V. Electroresponsive Polyelectrolyte Brushes Studied by Self-Consistent Field Theory. Polymers 2020, 12, 898. https://doi.org/10.3390/polym12040898
Okrugin BM, Richter RP, Leermakers FAM, Neelov IM, Zhulina EB, Borisov OV. Electroresponsive Polyelectrolyte Brushes Studied by Self-Consistent Field Theory. Polymers. 2020; 12(4):898. https://doi.org/10.3390/polym12040898
Chicago/Turabian StyleOkrugin, Boris M., Ralf P. Richter, Frans A. M. Leermakers, Igor M. Neelov, Ekaterina B. Zhulina, and Oleg V. Borisov. 2020. "Electroresponsive Polyelectrolyte Brushes Studied by Self-Consistent Field Theory" Polymers 12, no. 4: 898. https://doi.org/10.3390/polym12040898