Personalised 3D Printed Fast-Dissolving Tablets for Managing Hypertensive Crisis: In-Vitro/In-Vivo Studies
Abstract
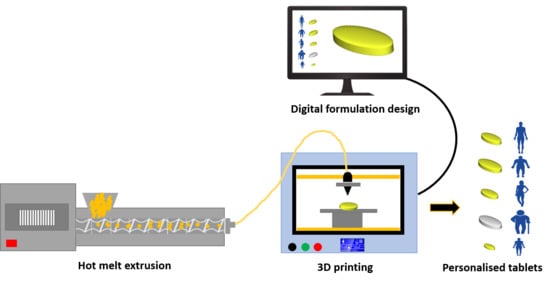
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Filaments
2.3. Physicochemical Characterisation of Filaments
2.3.1. Determination of Drug Loading
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. X-ray Powder Diffraction (XRPD)
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. Mechanical Testing
2.4. Fabrication of 3D Printed Tablets
2.5. Characterisation of 3D Printed Tablets
2.5.1. Geometrical, Porosity, and Morphological Assessment of 3D Printed Tablets
2.5.2. Determination of Tablet Hardness and Friability
2.5.3. 3D parametric Surface Texture Analysis
2.5.4. In-Vitro Disintegration Testing
2.5.5. In-Vitro Captopril Dissolution Investigation
2.5.6. In-Vivo Pharmacokinetic Investigation
3. Results and Discussion
3.1. Development and Characterisation of Filaments
3.2. Development and Characterisation of 3D Printed Matrix Tablets
3.2.1. Content Uniformity, Hardness, Porosity, and Friability of Printed Tablets
3.2.2. Morphological Analysis of Printed Tablets
3.2.3. 3D Parametric Surface Texture Analysis of 3D Printed Tablets
3.2.4. In-Vitro Disintegration and Dissolution Studies
3.2.5. In-Vivo Pharmacokinetic Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Mittal, B.V.; Singh, A.K. Hypertension in the developing world: Challenges and opportunities. Am. J. Kidney Dis. 2010, 55, 590–598. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Coates, M.M.; Cohen, A.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef] [Green Version]
- Khajedaluee, M.; Hassannia, T.; Rezaee, A.; Ziadi, M.; Dadgarmoghaddam, M. The prevalence of hypertension and its relationship with demographic factors, biochemical, and anthropometric indicators: A population-based study. ARYA Atheroscler. 2016, 12, 259–265. [Google Scholar] [PubMed]
- Dzau, V.J.; Balatbat, C.A. Future of Hypertension. Hypertension 2019, 74, 450–457. [Google Scholar] [CrossRef]
- Aggarwal, M.; Khan, I.A. Hypertensive crisis: Hypertensive emergencies and urgencies. Cardiol. Clin. 2006, 24, 135–146. [Google Scholar] [CrossRef]
- Rodriguez, M.A.; Kumar, S.K.; De Caro, M. Hypertensive crisis. Cardiol. Rev. 2010, 18, 102–107. [Google Scholar] [CrossRef]
- Seeman, T.; Hamdani, G.; Mitsnefes, M. Hypertensive crisis in children and adolescents. Pediatr. Nephrol. 2019, 34, 2523–2537. [Google Scholar] [CrossRef]
- Varon, J. Treatment of acute severe hypertension: Current and newer agents. Drugs 2008, 68, 283–297. [Google Scholar] [CrossRef]
- Salkic, S.; Batic-Mujanovic, O.; Ljuca, F.; Brkic, S. Clinical presentation of hypertensive crises in emergency medical services. Mater. Sociomed. 2014, 26, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Calhoun, D.A.; Oparil, S. Treatment of hypertensive crisis. N. Engl. J. Med. 1990, 323, 1177–1183. [Google Scholar] [PubMed]
- Varon, J. The diagnosis and treatment of hypertensive crises. J. Postgrad. Med. 2009, 121, 5–13. [Google Scholar] [CrossRef] [PubMed]
- The fifth report of the joint national committee on detection, evaluation, and treatment of high blood pressure (JNC V). Arch. Intern. Med. 1993, 153, 154–183. [CrossRef]
- Nur, A.O.; Zhang, J.S. Recent progress in sustained/ controlled oral delivery of captopril: An overview. Int. J. Pharm. 2000, 194, 139–146. [Google Scholar] [CrossRef]
- Ohman, K.P.; Kagedal, B.; Larsson, R.; Kalberg, E.B. Pharmacokinetics of captopril and its effect on blood pressure during acute and chronic administration and in relation to food intake. J. Cardiovasc. Pharm. 1985, 7, S20–S24. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.F.; Liu, F.; Brown, M.B. Advances in oral transmucosal drug delivery. J. Control. Release 2011, 153, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chow, M.S. Overview and appraisal of the current concept and technologies for improvement of sublingual drug delivery. Ther. Deliv. 2014, 5, 807–816. [Google Scholar] [CrossRef]
- Sattar, M.; Sayed, O.M.; Lane, M.E. Oral transmucosal drug delivery–current status and future prospects. Int. J. Pharm. 2014, 471, 498–506. [Google Scholar] [CrossRef]
- Baltzley, S.; Malkawi, A.A.; Alsmadi, M.; Al-Ghananeem, A.M. Sublingual spray drug delivery of ketorolac-loaded chitosan nanoparticles. Drug Dev. Ind. Pharm. 2018, 44, 1467–1472. [Google Scholar] [CrossRef]
- Rezaee, F.; Ganji, F. Formulation, Characterization, and Optimization of Captopril Fast-Dissolving Oral Films. AAPS PharmSciTech 2018, 19, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.; Gardouh, A.R.; Abogresha, N.M.; Gad, S. Factorial design, formulation, in vitro and in vivo evaluation of rapid orally disintegrating tablets prepared by sublimation technique using captopril as a model drug. J. Drug Deliv. Sci. Technol. 2020, 57, 101635. [Google Scholar] [CrossRef]
- Musuc, A.M.; Anuta, V.; Atkinson, I.; Popa, V.T.; Sarbu, I.; Mircioiu, C.; Abdalrb, G.A.; Mitu, M.A.; Ozon, E.A. Development and Characterization of Orally Disintegrating Tablets Containing a Captopril-Cyclodextrin Complex. Pharmaceutics 2020, 12, 744. [Google Scholar] [CrossRef] [PubMed]
- Ghori, M.U.; Nirwan, J.S.; Asim, T.; Chahid, Y.; Farhaj, S.; Khizer, Z.; Timmins, P.; Conway, B.R. MUCO-DIS: A New AFM-Based Nanoscale Dissolution Technique. AAPS PharmSciTech 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Senjoti, F.G.; Ghori, M.U.; Diryak, R.; Conway, B.R.; Morris, G.A.; Smith, A.M. Rheo-dissolution: A new platform for the simultaneous measurement of rheology and drug release. Carbohydr. Polym. 2020, 229, 115541. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, F.; Hussain, A.; Arshad, M.S.; Abbas, N.; Irfan, M.; Qamar, N.; Ghori, M.U. Effect of Solublising Aids on the Entrapment of Loratidine in Pre-Fabricated PVA Filaments used for FDM Based 3D-Printing. Acta Pol. Pharm. 2020, 77, 175–182. [Google Scholar] [CrossRef]
- Qamar, N.; Abbas, N.; Irfan, M.; Hussain, A.; Arshad, M.S.; Latif, S.; Ghori, M.U. Personalized 3D printed ciprofloxacin impregnated meshes for the management of hernia. J. Drug Deliv. Sci. Technol. 2019, 53, 101164. [Google Scholar] [CrossRef]
- Khizer, Z.; Akram, M.R.; Sarfraz, R.M.; Nirwan, J.S.; Farhaj, S.; Yousaf, M.; Hussain, T.; Lou, S.; Timmins, P.; Conway, B.R.; et al. Plasticiser-free 3D printed hydrophilic matrices: Quantitative 3D surface texture, mechanical, swelling, erosion, drug release and pharmacokinetic studies. Polymers 2019, 11, 1095. [Google Scholar] [CrossRef] [Green Version]
- Lou, S.; Pagani, L.; Zeng, W.; Ghori, M.U.; Jiang, X.; Scott, P.J. Surface texture evaluation of additively manufactured metallic cellular scaffolds for acetabular implants using X-ray computed tomography. BDM Bio-Des. Manuf. 2019, 2, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Rudrangi, S.R.S.; Kaialy, W.; Ghori, M.U.; Trivedi, V.; Snowden, M.J.; Alexander, B.D. Solid-state flurbiprofen and methyl-β-cyclodextrin inclusion complexes prepared using a single-step, organic solvent-free supercritical fluid process. Eur. J. Pharm. Biopharm. 2016, 104, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, Y.; Saeed, S.; Ghori, M.U.; Mahmood, T.; Yousaf, A.M.; Jamshaid, M.; Rizvi, S.A. Influence of polymer ratio and surfactants on controlled drug release from cellulosic microsponges. Int. J. Biol. Macromol. 2018, 109, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Diryak, R.; Kontogiorgos, V.; Ghori, M.U.; Bills, P.; Tawfik, A.; Morris, G.A.; Smith, A.M. Behavior of In Situ Cross-Linked Hydrogels with Rapid Gelation Kinetics on Contact with Physiological Fluids. Macromol. Chem. Phys. 2018, 219, 1700584. [Google Scholar] [CrossRef]
- Nirwan, J.S.; Conway, B.R.; Ghori, M.U. In situ 3D nanoscale advanced imaging algorithms with integrated chemical imaging for the characterisation of pharmaceuticals. RSC Adv. 2019, 9, 16119–16129. [Google Scholar] [CrossRef] [Green Version]
- Khizer, Z.; Nirwan, J.S.; Conway, B.R.; Ghori, M.U. Okra (Hibiscus esculentus) gum based hydrophilic matrices for controlled drug delivery applications: Estimation of percolation threshold. Int. J. Biol. Macromol. 2020, 155, 835–845. [Google Scholar] [CrossRef]
- Allam, A.; Fetih, G. Sublingual fast dissolving niosomal films for enhanced bioavailability and prolonged effect of metoprolol tartrate. Drug Des. Dev. Ther. 2016, 10, 2421. [Google Scholar]
- IqbaL, F.M.; Ahmad, M.; Zubair, M.M.; Tulain, U.R.; Rashid, A. Determination of Captopril in Plasma by High-Performance Liquid Chromatography: Application in an In-Vivo Evaluation of Drug Release from Hydrogel. Lat. Am. J. Pharm. 2015, 34, 5. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Ghori, M.U.; Šupuk, E.; Conway, B.R. Tribo-electrification and powder adhesion studies in the development of polymeric hydrophilic drug matrices. Materials 2015, 8, 1482–1498. [Google Scholar] [CrossRef] [Green Version]
- Ghori, M.U. Release Kinetics, Compaction and Electrostatic Properties of Hydrophilic Ghori. Ph.D. Thesis, Repository University of Huddersfield, Huddersfield, UK, December 2014; p. 37. [Google Scholar]
- Aho, J.; Bøtker, J.P.; Genina, N.; Edinger, M.; Arnfast, L.; Rantanen, J. Roadmap to 3D-printed oral pharmaceutical dosage forms: Feedstock filament properties and characterization for fused deposition modeling. J. Pharm. Sci. 2019, 108, 26–35. [Google Scholar] [CrossRef] [Green Version]
- British Pharmacopoeia (BP). Available online: https://www.pharmacopoeia.com/ (accessed on 15 October 2019).
- Zhang, J.; Yang, W.; Vo, A.Q.; Feng, X.; Ye, X.; Kim, D.W.; Repka, M.A. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: Structure and drug release correlation. Carbohydr. Polym. 2017, 177, 49–57. [Google Scholar] [CrossRef]
- Kempin, W.; Domsta, V.; Grathoff, G.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Immediate release 3D-printed tablets produced via fused deposition modeling of a thermo-sensitive drug. Pharm. Res. 2018, 35, 124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]









| Formulation | Tensile Strength (MPa) | Young’s Modulus (MPa) | Stiffness (kN/m) | Drug Loading (%) |
|---|---|---|---|---|
| F1 | 20.13 ± 0.38 | 1931.5 ± 193.1 | 134.8 ± 11.8 | - |
| F2 | 19.81 ± 0.25 | 1727.8 ± 434.3 | 111.7 ± 26.6 | 98.65 ± 0.77 |
| F3 | 19.95 ± 0.34 | 1468.9 ± 109.8 | 89.97 ± 6.72 | 97.43 ± 1.2 |
| F4 | 20.16 ± 0.26 | 1475.3 ± 279.6 | 86.48 ± 5.2 | 96.50 ± 0.35 |
| Formulation | Weight (mg) | Diameter (mm) | Height (mm) | Drug Loading (%) | Breaking Strength (N) | Porosity (%) | Friability (%) |
|---|---|---|---|---|---|---|---|
| F1 | 175.0 ± 0.75 | 7.00 ± 0.10 | 2.13 ± 0.11 | - | 385.1 ± 11.5 | 1.6 ± 0.6 | 0 |
| F2 | 175.2 ± 0.80 | 7.05 ± 0.12 | 2.15 ± 0.15 | 97.15 ± 1.2 | 411.3 ± 19.3 | 2.4 ± 0.5 | 0 |
| F3 | 175.1 ± 1.1 | 7.02 ± 0.15 | 2.20 ± 0.10 | 96.77 ± 0.8 | 406.4 ± 21.3 | 2.1 ± 0.3 | 0 |
| F4 | 175.4 ± 0.5 | 7.10 ± 0.18 | 2.22 ± 0.12 | 98.10 ± 1.1 | 409.9 ± 6.98 | 1.7 ± 0.4 | 0 |
| Parameter | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Sa (μm) | 20.11 ± 4.25 | 24.19 ± 2.14 | 22.16 ± 1.54 | 25.19 ± 3.31 |
| Sq (μm) | 25.29 ± 6.84 | 30.15 ± 3.11 | 31.25 ± 2.88 | 33.22 ± 2.44 |
| Sz (μm) | 57.83 ± 5.92 | 61.95 ± 6.86 | 67.59 ± 5.12 | 75.95 ± 6.11 |
| Sp (μm) | 24.16 ± 4.21 | 39.26 ± 2.49 | 41.26 ± 4.16 | 44.29 ± 6.12 |
| Sv (μm) | 33.67 ± 5.22 | 22.69 ± 7.15 | 26.33 ± 7.19 | 31.66 ± 6.05 |
| Sku (μm) | 4.21 ± 0.95 | 4.55 ± 1.11 | 4.88 ± 0.89 | 5.11 ± 1.21 |
| Ssk (μm) | −0.414 ± −0.48 | −0.29 ± −0.11 | −0.42 ± −0.15 | −0.36 ± −0.10 |
| Parameter | Standard | F2 | F3 | F4 |
|---|---|---|---|---|
| Tmax (h) | 2.00 ± 0.00 | 3.67 ± 0.52 | 2.00 ± 0.00 | 2.17 ± 0.41 |
| Cmax (ng/mL) | 1158.45 ± 71.67 | 1054.12 ± 64.36 | 1117.02 ± 32.46 | 1175.11 ± 21.65 |
| AUC0–24h (ng/mL·h) | 6004.88 ± 125.49 | 7039.39 ± 931.58 | 5836.30 ± 102.51 | 4006.75 ± 58.66 |
| MRT (h) | 6.51 ± 0.76 | 11.98 ± 1.98 | 7.66 ± 0.28 | 4.70 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, A.; Mahmood, F.; Arshad, M.S.; Abbas, N.; Qamar, N.; Mudassir, J.; Farhaj, S.; Nirwan, J.S.; Ghori, M.U. Personalised 3D Printed Fast-Dissolving Tablets for Managing Hypertensive Crisis: In-Vitro/In-Vivo Studies. Polymers 2020, 12, 3057. https://doi.org/10.3390/polym12123057
Hussain A, Mahmood F, Arshad MS, Abbas N, Qamar N, Mudassir J, Farhaj S, Nirwan JS, Ghori MU. Personalised 3D Printed Fast-Dissolving Tablets for Managing Hypertensive Crisis: In-Vitro/In-Vivo Studies. Polymers. 2020; 12(12):3057. https://doi.org/10.3390/polym12123057
Chicago/Turabian StyleHussain, Amjad, Faisal Mahmood, Muhammad Sohail Arshad, Nasir Abbas, Nadia Qamar, Jahanzeb Mudassir, Samia Farhaj, Jorabar Singh Nirwan, and Muhammad Usman Ghori. 2020. "Personalised 3D Printed Fast-Dissolving Tablets for Managing Hypertensive Crisis: In-Vitro/In-Vivo Studies" Polymers 12, no. 12: 3057. https://doi.org/10.3390/polym12123057