Polydimethylsiloxane Elastomers Filled with Rod-Like α-MnO2 Nanoparticles: An Interplay of Structure and Electrorheological Performance
Abstract
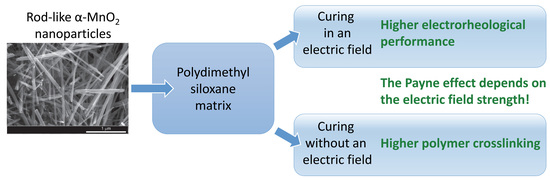
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Manganese Dioxide Nanorods (α-MnO2)
2.2. Preparation of Elastomeric Composites
2.3. Evaluation of the Degree of Crosslinking of the Elastomer
2.4. Methods of Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shiga, T.; Ohta, T.; Hirose, Y.; Okada, A.; Kurauchi, T. Electroviscoelastic effect of polymeric composites consisting of polyelectrolyte particles and polymer gel. J. Mater. Sci. 1993, 28, 1293–1299. [Google Scholar] [CrossRef]
- Shiga, T. Deformation and viscoelastic behavior of polymer gels in electric fields. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 1998, 74, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, J.; Li, W.; Du, H. A state-of-the-art review on magnetorheological elastomer devices. Smart Mater. Struct. 2014, 23, 123001. [Google Scholar] [CrossRef]
- Dong, X.; Niu, C.; Qi, M. Enhancement of electrorheological performance of electrorheological elastomers by improving TiO2 particles/silicon rubber interface. J. Mater. Chem. C 2016, 4, 6806–6815. [Google Scholar] [CrossRef]
- Niu, C.; Dong, X.; Qi, M. Enhanced Electrorheological Properties of Elastomers Containing TiO2 /Urea Core–Shell Particles. ACS Appl. Mater. Interfaces 2015, 7, 24855–24863. [Google Scholar] [CrossRef]
- Kossi, A.; Bossis, G.; Persello, J. Electro-active elastomer composites based on doped titanium dioxide. J. Mater. Chem. C 2015, 3, 1546–1556. [Google Scholar] [CrossRef]
- Dong, X.; Niu, C.; Qi, M. Electrorheological Elastomers. In Elastomers; InTech: London, UK, 2017. [Google Scholar]
- Rajhan, N.H.; Hamid, H.A.; Azmi, I.; Ismail, R. Magnetorheological Elastomers: A Review. Appl. Mech. Mater. 2014, 695, 255–259. [Google Scholar] [CrossRef]
- Tangboriboon, N.; Sirivat, A.; Kunanuruksapong, R.; Wongkasemjit, S. Electrorheological properties of novel piezoelectric lead zirconate titanate Pb(Zr0.5,Ti0.5)O3-acrylic rubber composites. Mater. Sci. Eng. C 2009, 29, 1913–1918. [Google Scholar] [CrossRef]
- Puvanatvattana, T.; Chotpattananont, D.; Hiamtup, P.; Niamlang, S.; Sirivat, A.; Jamieson, A.M. Electric field induced stress moduli in polythiophene/polyisoprene elastomer blends. React. Funct. Polym. 2006, 66, 1575–1588. [Google Scholar] [CrossRef]
- Thongsak, K.; Kunanuruksapong, R.; Sirivat, A.; Lerdwijitjarud, W. Electroactive polydiphenylamine/poly(styrene-block-isoprene-block-styrene) (SIS) blends: Effects of particle concentration and electric field. Mater. Sci. Eng. C 2011, 31, 206–214. [Google Scholar] [CrossRef]
- Kunanuruksapong, R.; Sirivat, A. Poly(p-phenylene) and acrylic elastomer blends for electroactive application. Mater. Sci. Eng. A 2007, 454–455, 453–460. [Google Scholar] [CrossRef]
- Kunanuruksapong, R.; Sirivat, A. Highly Electroresponsive Polymer Blends of Polyaniline Nanoparticles and Chloroprene Rubbers. Adv. Polym. Technol. 2013, 32, E556–E571. [Google Scholar] [CrossRef]
- Hiamtup, P.; Sirivat, A.; Jamieson, A.M. Electromechanical response of a soft and flexible actuator based on polyaniline particles embedded in a cross-linked poly(dimethyl siloxane) network. Mater. Sci. Eng. C 2008, 28, 1044–1051. [Google Scholar] [CrossRef]
- Liu, Y.D.; Choi, H.J. Electrorheological fluids: Smart soft matter and characteristics. Soft Matter 2012, 8, 11961–11978. [Google Scholar] [CrossRef]
- Parthasarathy, M.; Klingenberg, D.J. Electrorheology: Mechanisms and models. Mater. Sci. Eng. R Rep. 1996, 17, 57–103. [Google Scholar] [CrossRef]
- Hao, T. Electrorheological suspensions. Adv. Colloid Interface Sci. 2002, 97, 1–35. [Google Scholar] [CrossRef]
- Klingenberg, D.J.; Zukoski, C.F. Studies on the steady-shear behavior of electrorheological suspensions. Langmuir 1990, 6, 15–24. [Google Scholar] [CrossRef]
- Kim, Y.D.; Klingenberg, D.J. An interfacial polarization model for activated electrorheological suspensions. Korean J. Chem. Eng. 1997, 14, 30–36. [Google Scholar] [CrossRef]
- Kunanuruksapong, R.; Sirivat, A. Electrical properties and electromechanical responses of acrylic elastomers and styrene copolymers: Effect of temperature. Appl. Phys. A 2008, 92, 313–320. [Google Scholar] [CrossRef]
- Ludeelerd, P.; Niamlang, S.; Kunaruksapong, R.; Sirivat, A. Effect of elastomer matrix type on electromechanical response of conductive polypyrrole/elastomer blends. J. Phys. Chem. Solids 2010, 71, 1243–1250. [Google Scholar] [CrossRef]
- Liu, B.; Shaw, M.T. Electrorheology of filled silicone elastomers. J. Rheol. 2001, 45, 641–657. [Google Scholar] [CrossRef]
- Shen, R.; Wang, X.; Lu, Y.; Wang, D.; Sun, G.; Cao, Z.; Lu, K. Polar-Molecule-Dominated Electrorheological Fluids Featuring High Yield Stresses. Adv. Mater. 2009, 21, 4631–4635. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, J.; Xu, G.; Cui, P.; Liu, X.; Liu, F.; Wu, J. Electrorheological property and microstructure of acetamide-modified TiO2 nanoparticles. Colloid Polym. Sci. 2008, 286, 1493–1497. [Google Scholar] [CrossRef]
- Cao, J.G.; Shen, M.; Zhou, L.W. Preparation and electrorheological properties of triethanolamine-modified TiO2. J. Solid State Chem. 2006, 179, 1565–1568. [Google Scholar] [CrossRef]
- Davydova, O.I.; Kraev, A.S.; Redozubov, A.A.; Trusova, T.A.; Agafonov, A.V. Effect of polydimethylsiloxane viscosity on the electrorheological activity of dispersions based on it. Russ. J. Phys. Chem. A 2016, 90, 1269–1273. [Google Scholar] [CrossRef]
- Boczkowska, A. (Ed.) Advanced Elastomers—Technology, Properties and Applications; InTech: London, UK, 2012; ISBN 978-953-51-0739-2. [Google Scholar]
- Puente-Córdova, J.; Reyes-Melo, M.; Palacios-Pineda, L.; Martínez-Perales, I.; Martínez-Romero, O.; Elías-Zúñiga, A. Fabrication and Characterization of Isotropic and Anisotropic Magnetorheological Elastomers, Based on Silicone Rubber and Carbonyl Iron Microparticles. Polymers 2018, 10, 1343. [Google Scholar] [CrossRef] [Green Version]
- Agafonov, A.V.; Kraev, A.S.; Kusova, T.V.; Evdokimova, O.L.; Ivanova, O.S.; Baranchikov, A.E.; Shekunova, T.O.; Kozyukhin, S.A. Surfactant-Switched Positive/Negative Electrorheological Effect in Tungsten Oxide Suspensions. Molecules 2019, 24, 3348. [Google Scholar] [CrossRef] [Green Version]
- Agafonov, A.V.; Krayev, A.S.; Davydova, O.I.; Ivanov, K.V.; Shekunova, T.O.; Baranchikov, A.E.; Ivanova, O.S.; Borilo, L.P.; Garshev, A.V.; Kozik, V.V.; et al. Nanocrystalline ceria: A novel material for electrorheological fluids. RSC Adv. 2016, 6, 88851–88858. [Google Scholar] [CrossRef]
- Agafonov, A.V.; Kraev, A.S.; Ivanova, O.S.; Evdokimova, O.L.; Gerasimova, T.V.; Baranchikov, A.E.; Kozik, V.V.; Ivanov, V.K. Comparative study of the electrorheological effect in suspensions of needle-like and isotropic cerium dioxide nanoparticles. Rheol. Acta 2018, 57, 307–315. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Kraev, A.S.; Gaitko, O.M.; Gerasimova, T.V.; Golodukhina, S.V.; Agafonov, A.V. Electrorheological Properties of α-Bi2O3 and Bi2O2CO3. Inorg. Mater. 2019, 55, 344–354. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Kraev, A.S.; Gajtko, O.M.; Baranchikov, A.E.; Agafonov, A.V.; Ivanov, V.K. Electrorheological Fluids Based on Bismuth Ferrites BiFeO3 and Bi2Fe4O9. Russ. J. Inorg. Chem. 2020, 65, 1253–1263. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Kraev, A.S.; Gajtko, O.M.; Kusova, T.V.; Baranchikov, A.E.; Agafonov, A.V.; Ivanov, V.K. High electrorheological effect in Bi1.8Fe1.2SbO7 suspensions. Powder Technol. 2020, 360, 96–103. [Google Scholar] [CrossRef]
- John, R.E.; Chandran, A.; Samuel, M.; Thomas, M.; George, K.C. Origin of colossal dielectric behavior in hydrothermally prepared non-stoichiometric α-MnO2 nanorods. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 116, 113720. [Google Scholar] [CrossRef]
- Agafonov, A.V.; Kraev, A.S.; Teplonogova, M.A.; Baranchikov, A.E.; Ivanov, V.K. First MnO2-based electrorheological fluids: High response at low filler concentration. Rheol. Acta 2019, 58, 719–728. [Google Scholar] [CrossRef]
- Subramanian, V.; Zhu, H.; Vajtai, R.; Ajayan, P.M.; Wei, B. Hydrothermal synthesis and pseudocapacitance properties of MnO2 nanostructures. J. Phys. Chem. B 2005, 109, 20207–20214. [Google Scholar] [CrossRef]
- Hashemzadeh, F.; Mehdi Kashani Motlagh, M.; Maghsoudipour, A. A comparative study of hydrothermal and sol–gel methods in the synthesis of MnO2 nanostructures. J. Sol-Gel Sci. Technol. 2009, 51, 169–174. [Google Scholar] [CrossRef]
- Mazurek, P.; Vudayagiri, S.; Skov, A.L. How to tailor flexible silicone elastomers with mechanical integrity: A tutorial review. Chem. Soc. Rev. 2019, 48, 1448–1464. [Google Scholar] [CrossRef] [Green Version]
- Zhukov, A.V.; Mushenko, V.D.; Baratova, T.N. Catalytic Mixture for Hardening Siloxane Rubber. RU Patent RU2424260, 20 July 2011. [Google Scholar]
- Manaila, E.; Stelescu, M.D.; Craciun, G.; Surdu, L. Effects of benzoyl peroxide on some properties of composites based on hemp and natural rubber. Polym. Bull. 2014, 71, 2001–2022. [Google Scholar] [CrossRef]
- Ogieglo, W.; van der Werf, H.; Tempelman, K.; Wormeester, H.; Wessling, M.; Nijmeijer, A.; Benes, N.E. n-Hexane induced swelling of thin PDMS films under non-equilibrium nanofiltration permeation conditions, resolved by spectroscopic ellipsometry. J. Memb. Sci. 2013, 437, 313–323. [Google Scholar] [CrossRef]
- Kappert, E.J.; Raaijmakers, M.J.T.; Tempelman, K.; Cuperus, F.P.; Ogieglo, W.; Benes, N.E. Swelling of 9 polymers commonly employed for solvent-resistant nanofiltration membranes: A comprehensive dataset. J. Memb. Sci. 2019, 569, 177–199. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Cai, C.; Liu, W.; Li, D.; Zhang, J.; Zhao, N.; Xu, J. Recyclable Polydimethylsiloxane Network Crosslinked by Dynamic Transesterification Reaction. Sci. Rep. 2017, 7, 11833. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, S.H. New technology of crumb rubber compounding for recycling of waste tires. J. Appl. Polym. Sci. 2000, 78, 1573–1577. [Google Scholar] [CrossRef]
- Valentiín, J.L.; Carretero-Gonzaález, J.; Mora-Barrantes, I.; Chasseé, W.; Saalwachter, K. Uncertainties in the Determination of Cross-Link Density by Equilibrium Swelling Experiments in Natural Rubber. Macromolecules 2008, 41, 4717–4729. [Google Scholar] [CrossRef]
- Birgisson, S.; Saha, D.; Iversen, B.B. Formation Mechanisms of Nanocrystalline MnO2 Polymorphs under Hydrothermal Conditions. Cryst. Growth Des. 2018, 18, 827–838. [Google Scholar] [CrossRef]
- Grigorieva, A.V.; Melnik, D.M.; Goodilin, E.A.; Anufrieva, T.A.; Derlyukova, L.E.; Tretyakov, Y.D. Nanorods of cryptomelane via soft chemistry method and their catalytic activity. Solid State Sci. 2012, 14, 988–995. [Google Scholar] [CrossRef]
- Agafonov, A.V.; Kraev, A.S.; Baranchikov, A.E.; Ivanov, V.K. Electrorheological Properties of Polydimethylsiloxane/TiO2-Based Composite Elastomers. Polymers 2020, 12, 2137. [Google Scholar] [CrossRef]
- Zhao, J.; Milanova, M.; Warmoeskerken, M.M.C.G.; Dutschk, V. Surface modification of TiO2 nanoparticles with silane coupling agents. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 413, 273–279. [Google Scholar] [CrossRef]
- Cui, X.; Zhu, G.; Pan, Y.; Shao, Q.; Zhao, C. (xinxin); Dong, M.; Zhang, Y.; Guo, Z. Polydimethylsiloxane-titania nanocomposite coating: Fabrication and corrosion resistance. Polymer 2018, 138, 203–210. [Google Scholar] [CrossRef]
- Wen, J.; Mark, J.E. Sol–Gel Preparation of Composites of Poly(dimethylsiloxane) with SiO2 and SiO2/TiO2, and Their Mechanical Properties. Polym. J. 1995, 27, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.J.; Hong, C.H.; Jhon, M.S. Cole-Cole Analysis on Dielectric Spectra of Electrorheological Suspensions. Int. J. Mod. Phys. B 2007, 21, 4974–4980. [Google Scholar] [CrossRef]
- Jonscher, A.K. The ‘universal’ dielectric response. Nature 1977, 267, 673–679. [Google Scholar] [CrossRef]
- Dueñas, J.M.M.; Mateo, J.M.; Ribelles, J.L.G. Influence of the cross-linking density on the main dielectric relaxation of poly(methyl acrylate) networks. Polym. Eng. Sci. 2005, 45, 1336–1342. [Google Scholar] [CrossRef]
- Yin, J.; Xia, X.; Wang, X.; Zhao, X. The electrorheological effect and dielectric properties of suspensions containing polyaniline@titania nanocable-like particles. Soft Matter 2011, 7, 10978. [Google Scholar] [CrossRef]
- Stěnička, M.; Pavlínek, V.; Sáha, P.; Blinova, N.V.; Stejskal, J.; Quadrat, O. The electrorheological efficiency of polyaniline particles with various conductivities suspended in silicone oil. Colloid Polym. Sci. 2009, 287, 403–412. [Google Scholar] [CrossRef]
- Lim, E.; Manaka, T.; Iwamoto, M. Analysis of pentacene field-effect transistor with contact resistance as an element of a Maxwell–Wagner effect system. J. Appl. Phys. 2008, 104, 054511. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook. For Users of Rotational and Oscillatory Rheometer, 4th ed.; Vincentz Network: Hanover, Germany, 2014; ISBN 978-3-86630-650-9. [Google Scholar]
- Li, R.; Sun, L.Z. Dynamic mechanical analysis of silicone rubber reinforced with multi-walled carbon nanotubes. Interact. Multiscale Mech. 2011, 4, 239–245. [Google Scholar] [CrossRef]
- Madsen, F.B.; Yu, L.; Daugaard, A.E.; Hvilsted, S.; Skov, A.L. A new soft dielectric silicone elastomer matrix with high mechanical integrity and low losses. RSC Adv. 2015, 5, 10254–10259. [Google Scholar] [CrossRef] [Green Version]
- Hentschke, R. The Payne effect revisited. Express Polym. Lett. 2017, 11, 278–292. [Google Scholar] [CrossRef]
- Shamonin, M.; Kramarenko, E.Y. Highly Responsive Magnetoactive Elastomers. In Novel Magnetic Nanostructures; Elsevier: Amsterdam, The Netherlands, 2018; pp. 221–245. [Google Scholar]
- Kwon, S.; Lee, J.; Choi, H. Magnetic Particle Filled Elastomeric Hybrid Composites and Their Magnetorheological Response. Materials 2018, 11, 1040. [Google Scholar] [CrossRef] [Green Version]





| Sample | Swelling Ratio β, % | Gel Fraction GF, % | Uncured Polymer Content | Degree of Crosslinking , mol/cm3 |
|---|---|---|---|---|
| MnO2-0 | 118 | 97.5 | 2.4 | 7.6 × 10−4 |
| MnO2-E | 188 | 96.9 | 3.0 | 3.3 × 10−4 |
| Sample | E, kV/mm | |||
|---|---|---|---|---|
| 0.0 | 0.4 | 1.2 | 2.0 | |
| MnO2-0 | 4.6 | 1.5 | 1.5 | 1.4 |
| MnO2-E | 4.7 | 3.8 | 3.7 | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agafonov, A.V.; Kraev, A.S.; Egorova, A.A.; Baranchikov, A.E.; Kozyukhin, S.A.; Ivanov, V.K. Polydimethylsiloxane Elastomers Filled with Rod-Like α-MnO2 Nanoparticles: An Interplay of Structure and Electrorheological Performance. Polymers 2020, 12, 2810. https://doi.org/10.3390/polym12122810
Agafonov AV, Kraev AS, Egorova AA, Baranchikov AE, Kozyukhin SA, Ivanov VK. Polydimethylsiloxane Elastomers Filled with Rod-Like α-MnO2 Nanoparticles: An Interplay of Structure and Electrorheological Performance. Polymers. 2020; 12(12):2810. https://doi.org/10.3390/polym12122810
Chicago/Turabian StyleAgafonov, Alexander V., Anton S. Kraev, Anastasia A. Egorova, Alexander E. Baranchikov, Sergey A. Kozyukhin, and Vladimir K. Ivanov. 2020. "Polydimethylsiloxane Elastomers Filled with Rod-Like α-MnO2 Nanoparticles: An Interplay of Structure and Electrorheological Performance" Polymers 12, no. 12: 2810. https://doi.org/10.3390/polym12122810