Influence of an Alkoxylation Grade of Acrylates on Shrinkage of UV-Curable Compositions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparing of Tested Restorative Compositions
2.3. Shrinkage Measurement
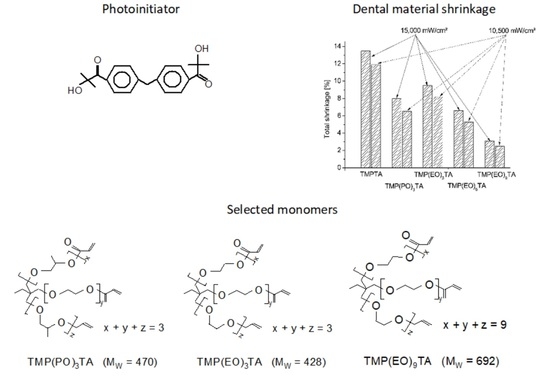
3. Results
Shrinkage Evaluation as a Function of Alkoxylated Diacrylates Kind and Amount
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Giachetti, L.; Russo, D.; Bambi, C.; Grandini, R. A review of polymerization shrinkage stress: Current techniques for posterior direct resin restorations. J. Contemp. Dent. Pract 2006, 7, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Santis, R.; Russo, T.; Gloria, A. An analysis on the potential of diode-pumped solid-state lasers for dental materials. Mater. Sci. Eng. C 2018, 92, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.L. Adhesive bonding of various materials to hard tissues. VI. Forces developing in direct-filling materials during hardening. J. Am. Dent. Assoc. 1967, 74, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Salz, U. New developments of polymeric dental composites. Prog. Polym. Sci. 2001, 26, 535–576. [Google Scholar] [CrossRef]
- Labella, R.; Lambrechts, P.; van Meerbeek, B.; Vanherle, G. Polymerization shrinkage and elasticity of flowable composites and filled adhesives. Dent. Mater. 1999, 15, 128–137. [Google Scholar] [CrossRef]
- Lim, B.-S.; Ferracane, J.L.; Sakaguchi, R.L.; Condon, J.R. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dent. Mater. 2002, 18, 436–444. [Google Scholar] [CrossRef]
- Lu, H.; Stansbury, J.W.; Bowman, C.N. Impact of curing protocol on conversion and shrinkage stress. J. Dent. Res. 2005, 84, 822–826. [Google Scholar] [CrossRef] [PubMed]
- De Santis, R.; Gloriaa, A.; Prisco, D.; Amendola, E.; Puppulin, L.; Pezzotti, G.; Rengo, S.; Ambrosio, L.; Nicolais, L. Fast curing of restorative materials through the soft light energy release. Dent. Mater. 2010, 26, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Dauvillier, B.S.; Feilzer, A.J.; de Gee, A.J.; Davidson, C.L. Viscoelastic parameters of dental restorative materials during setting. J. Dent. Res. 2000, 79, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Salz, U. Recent developments of new components for dental adhesives and composites. Macromol. Mater. Eng. 2007, 292, 245–271. [Google Scholar] [CrossRef]
- Braga, R.R.; Ferracane, J.L. Alternatives in polymerization contraction stress management. Crit. Rev. Oral Biol. Med. 2004, 15, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Landuyt, K.L.; Snauwaert, J.; de Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Moszner, N.; Hirt, T. New polymer-chemical developments in clinical dental polymer materials: Enamel-dentin adhesives and restorative composites. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4369–4402. [Google Scholar] [CrossRef]
- Venhoven, B.A.; de Gee, A.J.; Davidson, C.L. Polymerization contraction and conversion of light-curing bis GMA-based methacrylate resins. Biomaterials 1993, 14, 871–875. [Google Scholar] [CrossRef]
- Davidson, C.L.; Feilzer, A.J. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J. Dent. 1997, 25, 435–440. [Google Scholar] [CrossRef]
- Czech, Z.; Butwin, A.; Kabatc, J.; Świderska, J. UV-crosslinkable acrylic pressure-sensitive adhesives for industrial application. Polym. Bull. 2012, 69, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Czech, Z. Studies of photoreactive acrylic adhesives with high shrinkage resistance. Pol. J. Chem. Technol. 2004, 4, 5–9. [Google Scholar]
- Czech, Z.; Kowalczyk, A.; Kabatc, J.; Świderska, J. Photoreactive UV-crosslinkable solvent-free acrylic pressure-sensitive adhesives containing copolymerizable photoinitiators based on benzophenones. Eur. Polym. J. 2012, 48, 1446–1454. [Google Scholar] [CrossRef]
- Czech, Z. Solvent based pressure-sensitive adhesives for PVC surfaces. Adv. Polym. Technol. 2001, 20, 72–85. [Google Scholar] [CrossRef]
- Czech, Z. Solvent based pressure-sensitive adhesives for PVC sign and marking films. J. Appl. Polym. Sci. 2001, 81, 3212–3219. [Google Scholar] [CrossRef]







| Raw Material | Chemical Structure | Chemical Name |
|---|---|---|
| Hydroxylapatite | Ca5(PO4)3(OH) | mineral form of calcium apatite |
| Omnirad 127 |  | 2-hydroxy-1-{4-[4-(2-hydroxy-2-methyl-propionyl)-benzyl]-phenyl}-2-methyl-propan-1-one |
| Monomer | Chemical Structure | Producer | MW [Dalton] |
|---|---|---|---|
| Miramer M300 |  | Rahn USA (Aurora, IL) | 296 |
| Miramer M360 |  | Rahn USA (Aurora, IL) | 470 |
| Miramer M3130 |  | Rahn USA (Aurora, IL) | 428 |
| Miramer M3190 |  | Rahn USA (Aurora, IL) | 692 |
| Parameter | High Power Type 365 nm | High Power Type 385 nm |
|---|---|---|
| UV irradiation intensity | 10,500 mW/cm2 | 15,000 mW/cm2 |
| Peak wavelength | 365 ± 5 | 385 ± 5 |
| Monomer | Functionality | Molecular Weight [kg/kmol] | Density at 25 °C [kg/m3] | Concentration of Double Bonds Cdb [mol/L] | Maximal Shrinkage after UV Curing [%] |
|---|---|---|---|---|---|
| TMPTA | 3 | 296 | 1060 | 7.2 | 12.6 |
| TMP(PO)3TA | 3 | 470 | 1070 | 4.6 | 7.9 |
| TMP(EO)3TA | 3 | 428 | 1090 | 5.1 | 9.1 |
| TMP(PO)6TA | 3 | 560 | 1100 | 3.9 | 6.0 |
| TMP(PO)9TA | 3 | 692 | 1110 | 3.2 | 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czech, Z.; Kabatc, J.; Bartkowiak, M.; Mozelewska, K.; Kwiatkowska, D. Influence of an Alkoxylation Grade of Acrylates on Shrinkage of UV-Curable Compositions. Polymers 2020, 12, 2617. https://doi.org/10.3390/polym12112617
Czech Z, Kabatc J, Bartkowiak M, Mozelewska K, Kwiatkowska D. Influence of an Alkoxylation Grade of Acrylates on Shrinkage of UV-Curable Compositions. Polymers. 2020; 12(11):2617. https://doi.org/10.3390/polym12112617
Chicago/Turabian StyleCzech, Zbigniew, Janina Kabatc, Marcin Bartkowiak, Karolina Mozelewska, and Dominika Kwiatkowska. 2020. "Influence of an Alkoxylation Grade of Acrylates on Shrinkage of UV-Curable Compositions" Polymers 12, no. 11: 2617. https://doi.org/10.3390/polym12112617