Preparation and Optimization of Water-Soluble Cationic Sago Starch with a High Degree of Substitution Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
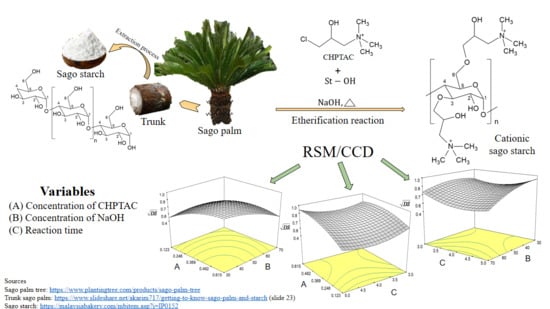
2.2. Preparation of Cationic Sago Starch
2.3. Design of Experiment and Response Surface Methodology
2.4. Determination of DS Value of Cationic Sago Starch
2.5. Characterization of Cationic Sago Starch
3. Results and Discussion
3.1. Data Analysis and Evaluation of the Model
3.1.1. Development of Regression Model Equation
3.1.2. Analysis of Variance, ANOVA
3.1.3. Effect of Independent Variables on the Values of DS
Effect of CHPTAC and NaOH Concentrations on DS
Effect of CHPTAC Concentration and Reaction Time on DS
Effect of NaOH Concentration and Reaction Time on DS
3.1.4. Validation of Model and Optimization of DS Value
3.2. Cationic Sago Starch Characterization
3.2.1. Fourier Transform Infrared, FTIR Spectroscopy Analysis
3.2.2. X-ray Diffraction, XRD Analysis
3.2.3. Field Emission Scanning Electron Microscopy, FESEM Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuo, W.Y.; Lai, H.M. Effects of reaction conditions on the physicochemical properties of cationic starch studied by RSM. Carbohydr. Polym. 2009, 75, 627–635. [Google Scholar] [CrossRef]
- Lim, W.J.; Liang, Y.T.; Seib, P.A. Cationic Oat Starch: Preparation and Effect on Paper Strength. Cereal Chem. 1992, 69, 237–239. [Google Scholar]
- Becerra, V.; Odermatt, J. Direct determination of cationic starches in paper samples using analytical pyrolysis. J. Anal. Appl. Pyrolysis 2014, 105, 348–354. [Google Scholar] [CrossRef]
- Santacruz, S. Characterisation of cationic potato starch by asymmetrical flow field-flow fractionation. Influence of ionic strength and degree of substitution. Carbohydr. Polym. 2014, 106, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Tajik, M.; Torshizi, H.J.; Resalati, H.; Hamzeh, Y. Effects of cationic starch in the presence of cellulose nanofibrils on structural, optical and strength properties of paper from soda bagasse pulp. Carbohydr. Polym. 2018, 194, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, K.; Asadpour, G.; Sharifi, S.H. Effect of coating with novel bio nanocomposites of cationic starch/cellulose nanocrystals on the fundamental properties of the packaging paper. Polym. Test. 2019, 80, 106080. [Google Scholar] [CrossRef]
- Khalil, M.I.; Aly, A.A. Use of cationic starch derivatives for the removal of anionic dyes from textile effluents. J. Appl. Polym. Sci. 2004, 93, 227–234. [Google Scholar] [CrossRef]
- Hebeish, A.; Aly, A.A.; El-Shafei, A.; Zaghloul, S. Synthesis and Characterization of Cationized Starches for Application in Flocculation, Finishing and Sizing. J. Chem. 2009, 52, 73–89. [Google Scholar]
- Wang, S.; Hou, Q.; Kong, F.; Fatehi, P. Production of cationic xylan-METAC copolymer as a flocculant for textile industry. Carbohydr. Polym. 2015, 124, 229–236. [Google Scholar] [CrossRef]
- Tomasik, P.; Schilling, C.H. Chemical Modification of Starch. Adv. Carbohydr. Chem. Biochem. 2004, 59, 175–403. [Google Scholar]
- Anthony, R.J. Cationic Starch Synthesis, Development, and Evaluation for Harvesting Microalgae for Wastewater Treatment. Utah State University: Logan, UT, USA, 2013; pp. 49–52. Available online: http://digitalcommons.usu.edu/etd/2005/ (accessed on 22 January 2019).
- Genest, S.; Petzold, G.; Schwarz, S. Removal of micro-stickies from model wastewaters of the paper industry by amphiphilic starch derivatives. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 231–241. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, M.; Gao, C.; Yang, J.; Zhang, X.; Zhang, X.; Liu, Z. Ultra-small and innocuous cationic starch nanospheres: Preparation, characterization and drug delivery study. Int. J. Biol. Macromol. 2013, 58, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, M.; Leichner, C.; Menzel, C.; Kreb, V.; Bernkop-Schnürch, A. Cationic starch derivatives as mucoadhesive and soluble excipients in drug delivery. Int. J. Pharm. 2019, 570, 118664. [Google Scholar] [CrossRef]
- Putro, J.N.; Ismadji, S.; Gunarto, C.; Soetaredjo, F.E.; Ju, Y.H. A study of anionic, cationic, and nonionic surfactants modified starch nanoparticles for hydrophobic drug loading and release. J. Mol. Liq. 2020, 298, 112034. [Google Scholar] [CrossRef]
- Khalil, M.I.; Aly, A.A. Preparation and evaluation of some cationic starch derivatives as flocculants. Starch Staerke 2001, 53, 84–89. [Google Scholar] [CrossRef]
- Siau, C.L.; Karim, A.A.; Norziah, M.H.; Wan Rosli, W.D. Effects of cationization on DSC thermal profiles, pasting and emulsifying properties of sago starch. J. Sci. Food Agric. 2004, 84, 1722–1730. [Google Scholar] [CrossRef]
- Heinze, T.; Haack, V.; Rensing, S. Starch derivatives of high degree of functionalization. 7. Preparation of cationic 2-hydroxypropyltrimethylammonium chloride starches. Starch Staerke 2004, 56, 288–296. [Google Scholar] [CrossRef]
- Bratskaya, S.; Schwarz, S.; Petzold, G.; Liebert, T.; Heinze, T. Cationic starches of high degree of functionalization: 12. Modification of cellulose fibers toward high filler technology in papermaking. Ind. Eng. Chem. Res. 2006, 45, 7374–7379. [Google Scholar] [CrossRef]
- Kuo, W.Y.; Lai, H.M. Changes of property and morphology of cationic corn starches. Carbohydr. Polym. 2007, 69, 544–553. [Google Scholar] [CrossRef]
- Liu, Q.X.; Li, J.L.; Xu, W.C. Preparation and properties of cationic starch with high degree of substitution. Mater. Sci. Forum 2011, 663–665, 1264–1267. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Hua, W.; Liu, W.H.; Wang, W.Q.; Ye, X.; Li, L.; Paul, C.; Roger, R. Synthesis and characterization of a starch-based cationic flocculant for microalgae harvesting. Int. J. Agric. Biol. Eng. 2016, 9, 139–145. [Google Scholar]
- Pfeifer, A.; Hampe, R.; Heinze, T. Synthesis and characterization of novel water-soluble and bactericidic cationic starch esters. Starch Staerke 2017, 69, 1700029. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, W. Synthesis of cationic starch with a high degree of substitution in an ionic liquid. Carbohydr. Polym. 2010, 80, 1172–1177. [Google Scholar] [CrossRef]
- Insel, P.M.; Turner, R.E.; Ross, D. Discovering Nutrition, 2nd ed.; Jones and Bartlett Learning Pr: Burlington, MA, USA; American Dietetic Association: Cleveland, OH, USA, 2006; p. 213. ISBN 13:9780763738990. [Google Scholar]
- Monohar, R.S. Chapter 8: Baking. In Conventional and Advanced Foods Processing Technologies; Battacharya, S., Ed.; John Wiley & Sons Ltd. Pr, Wiley Blackwell: Hoboken, NJ, USA, 2015; p. 170. ISBN 9781118406281. [Google Scholar]
- Khosroshahi, M.E. Properties of proteins. In Applications of Biophotonics and Nanobiomaterials in Biomedical Engineering; CRC Press Taylor&Francis Group A Science Pr: Boca Raton, FL, USA, 2017; p. 64. ISBN 9781315152202. [Google Scholar]
- Konuma, H. Status and outlook of global food security and the role of underutilized food resources: Sago Palm. In Sago Palm; Ehara, H., Toyoda, Y., Johnson, D., Eds.; Springer: Singapore, 2018; pp. 3–16. ISBN 978-981-10-5269-9. [Google Scholar]
- Talib, N.A.A.; Salam, F.; Yusof, N.A.; Alang Ahmad, S.A.; Sulaiman, Y. Modeling and optimization of electrode modified with poly(3,4-ethylenedioxythiophene)/graphene oxide composite by response surface methodology/Box-Behnken design approach. J. Electroanal. Chem. 2017, 787, 1–10. [Google Scholar] [CrossRef]
- Razali, M.A.A.; Sanusi, N.; Ismail, H.; Othman, N.; Ariffin, A. Application of response surface methodology (RSM) for optimization of cassava starch grafted polyDADMAC synthesis for cationic properties. Starch Staerke 2012, 64, 935–943. [Google Scholar] [CrossRef]
- Jiang, X.; Kuang, F.; Kong, F.; Yan, C. Prediction of the antiglycation activity of polysaccharides from Benincasa hispida using a response surface methodology. Carbohydr. Polym. 2016, 151, 358–363. [Google Scholar] [CrossRef]
- Hashem, M.; Refaie, R.; Hebeish, A. Crosslinking of partially carboxymethylated cotton fabric via cationization. J. Clean. Prod. 2005, 13, 947–954. [Google Scholar] [CrossRef]
- Zainal Abiddin, N.F.; Yusoff, A.; Ahmad, N. Optimisation of reaction conditions of octenyl succinic anhydride (OSA) modified sago starch using response surface methodology (RSM). Int. Food Res. J. 2015, 22, 930–935. [Google Scholar]
- Han, S.; Zhu, X.; Zhang, B. Optimization of reaction conditions of octenyl succinic anhydride potato starch and its morphology, crystalline structure and thermal characterization. Adv. Mater. Res. 2011, 236–238, 2279–2289. [Google Scholar] [CrossRef]
- Wang, P.X.; Wu, X.L.; Dong-Hua, X.; Kun, X.; Ying, T.; Xi-bang, D.; Wen-bo, L. Preparation and characterization of cationic corn starch with a high degree of substitution in dioxane-THF-water media. Carbohydr. Res. 2009, 344, 851–855. [Google Scholar]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the structural order of native starch granules using combined FTIR and XRD analysis. J. Polym. Res. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Sun, X.; Zhang, J.; Mi, Y.; Li, Q.; Guo, Z. Synthesis and antioxidant activity of cationic 1,2,3-Triazole functionalized starch derivatives. Polymers 2020, 12, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, K.; Liu, X.; Wang, W.; Yang, X.; Zhang, X. Synthesis of modified starch/polyvinyl alcohol composite for treating textile wastewater. Polymers 2020, 12, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czarnecka, E.; Nowaczyk, J. Semi-Natural Superabsorbents Based on Starch-g-poly(acrylic acid): Modification, Synthesis and Application. Polymers 2020, 12, 1794. [Google Scholar] [CrossRef]
- Jane, J. Current understanding on starch granule structures. J. Appl. Glycosci. 2006, 53, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Todica, M.; Nagy, E.M.; Niculaescu, C.; Stan, O.; Cioica, N.; Pop, C.V. XRD investigation of some thermal degraded starch based materials. J. Spectrosc. 2016, 1–6. [Google Scholar] [CrossRef]
- Tuan, M.N.F.A.-Z.; Zainuddin, N.; Ahmad@Ayob, M.; Tan, S.W. Preparation, optimization and swelling study of carboxymethyl sago starch (CMSS)–acid hydrogel. Chem. Cent. J. 2018, 12, 1–10. [Google Scholar]
- Singh, A.V.; Nath, L.K. Synthesis and evaluation of physicochemical properties of cross-linked sago starch. Int. J. Biol. Macromol. 2012, 50, 14–18. [Google Scholar] [CrossRef]
- Zainal Abiddin, N.F.; Yusoff, A.; Ahmad, N. Preparation and physicochemical properties of octenyl succinic anhydride (OSA) modified sago starch. Malays. J. Anal. Sci. 2016, 20, 806–811. [Google Scholar]







| Variables | Response (Experimental) | |||
|---|---|---|---|---|
| Sample | A: Conc. of CHPTAC (mol) | B: Conc. of NaOH (% w/v) | C: Reaction Time (h) | Degree of Substitution, DS |
| S1 | 0.123 | 30.0 | 3.00 | 0.56 |
| S2 | 0.615 | 30.0 | 3.00 | 0.91 |
| S3 | 0.123 | 70.0 | 3.00 | 0.67 |
| S4 | 0.615 | 70.0 | 3.00 | 0.62 |
| S5 | 0.123 | 30.0 | 5.00 | 0.62 |
| S6 | 0.615 | 30.0 | 5.00 | 1.10 |
| S7 | 0.123 | 70.0 | 5.00 | 0.45 |
| S8 | 0.615 | 70.0 | 5.00 | 0.60 |
| S9 | 0.123 | 50.0 | 4.00 | 0.55 |
| S10 | 0.615 | 50.0 | 4.00 | 0.73 |
| S11 | 0.369 | 30.0 | 4.00 | 0.72 |
| S12 | 0.369 | 70.0 | 4.00 | 0.60 |
| S13 | 0.369 | 50.0 | 3.00 | 0.86 |
| S14 | 0.369 | 50.0 | 5.00 | 0.83 |
| S15 | 0.369 | 50.0 | 4.00 | 0.78 |
| S16 | 0.369 | 50.0 | 4.00 | 0.79 |
| Source | Sum of Squares | D.O.F | Mean Square | F Value | Prob > F |
|---|---|---|---|---|---|
| Model | 0.396 | 9 | 0.044 | 28.57 | 0.0003 |
| A | 0.125 | 1 | 0.125 | 81.35 | 0.0001 |
| B | 0.097 | 1 | 0.096 | 62.75 | 0.0002 |
| C | 7.06 × 10−6 | 1 | 7.06 × 10−6 | 0.46 × 10−2 | * 0.9482 |
| A2 | 0.025 | 1 | 0.025 | 15.88 | 0.0072 |
| B2 | 0.016 | 1 | 0.016 | 10.16 | 0.0189 |
| C2 | 0.031 | 1 | 0.031 | 19.88 | 0.0043 |
| AB | 0.068 | 1 | 0.068 | 43.91 | 0.0006 |
| AC | 0.014 | 1 | 0.014 | 8.99 | 0.0241 |
| BC | 0.031 | 1 | 0.031 | 20.38 | 0.0040 |
| Lack of Fit | 0.92 × 10−2 | 5 | 0.18 × 10−2 | 90.81 | * 0.0795 |
| R2 | 0.9772 | ||||
| Adjusted R2 | 0.9430 | ||||
| Predicted R2 | 0.8178 | ||||
| Adeq. precision | 20.272 | ||||
| STD | 0.039 | ||||
| Exp. | Conc. of CHPTAC (mol) | Conc. Of NaOH (%w/v) | Reaction Time (h) | Residue Standard Error, RSE% | Reaction Efficiency, RE% | ||
|---|---|---|---|---|---|---|---|
| Predicted | Experiment | ||||||
| * 1 | 0.615 | 30.00 | 5.00 | 1.093 | 1.0936 | 0.05 | 100 |
| 2 | 0.600 | 30.00 | 4.99 | 1.084 | 1.1027 | 1.73 | 100 |
| 3 | 0.530 | 37.40 | 3.00 | 0.896 | 0.888 | 0.89 | 99.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md Nasir, N.; Abdulmalek, E.; Zainuddin, N. Preparation and Optimization of Water-Soluble Cationic Sago Starch with a High Degree of Substitution Using Response Surface Methodology. Polymers 2020, 12, 2614. https://doi.org/10.3390/polym12112614
Md Nasir N, Abdulmalek E, Zainuddin N. Preparation and Optimization of Water-Soluble Cationic Sago Starch with a High Degree of Substitution Using Response Surface Methodology. Polymers. 2020; 12(11):2614. https://doi.org/10.3390/polym12112614
Chicago/Turabian StyleMd Nasir, Nur’Izzah, Emilia Abdulmalek, and Norhazlin Zainuddin. 2020. "Preparation and Optimization of Water-Soluble Cationic Sago Starch with a High Degree of Substitution Using Response Surface Methodology" Polymers 12, no. 11: 2614. https://doi.org/10.3390/polym12112614