Hyperbranched Polyglycerols as Robust Up-Conversion Nanoparticle Coating Layer for Feasible Cell Imaging
Abstract
:1. Introduction
2. Experimental Part
2.1. Chemicals and Materials
2.2. Synthesis of hbPGs
2.3. Reductive Amination
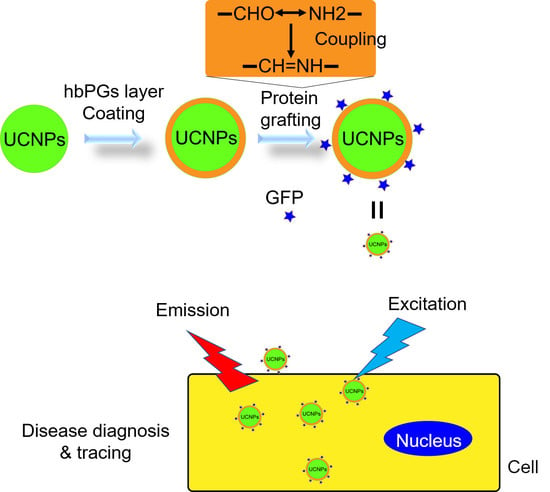
2.4. Preparation of hbPGs Grafting UCNP and Further Modification Process
2.5. NMR Titration
2.6. Dynamic Light Scattering (DLS) and Zeta Potential
2.7. Cell Culture and Cell Cytotoxicity
2.8. Drug-Loading Efficiency (DLE) and Drug Release Experiment
2.9. Confocal Laser Scanning Microscopy (CLSM) and Inverted Fluorescence Microscopy
3. Results and Discussion
3.1. Synthesis of Hyperbranched Polyglycerols (hbPGs)
3.2. Exploring the Modification Mechanism of hbPGs to React with Amino Acids
3.3. HbPGs Grafting UCNP Nanoparticles (hbPGs-g-UCNP-GFP)
4. Conclusions
5. Future Direction
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Son, S.; Shin, E.; Kim, B.-S. Light-responsive micelles of Spiropyran initiated hyperbranched polyglycerol for smart drug delivery. Biomacromolecules 2014, 15, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.I.; Kim, H.M.; Kim, J.H.; Moon, K.C.; Yoo, B.; Lee, K.T.; Lee, N.; Choi, Y.; Park, W.; Ling, D.; et al. Theranostic probe based on lanthanide-doped nanoparticles for simultaneous In Vivo dual-modal imaging and photodynamic therapy. Adv. Mater. 2012, 24, 5755–5761. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Y.; Yang, J.; Wei, P.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Hyaluronic acid-modified Fe3O4 @Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials 2015, 38, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Zhang, M. Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Accounts Chem. Res. 2011, 44, 853–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhang, Q.; Chang, H.; Cheng, Y. Surface-engineered dendrimers in gene delivery. Chem. Rev. 2015, 115, 5274–5300. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Schmidt, J.J.; Kohman, R.E.; Zill, A.T.; Devolder, R.J.; Smith, C.E.; Lai, M.-H.; Shkumatov, A.; Jensen, T.W.; Schook, L.G.; et al. Leukocyte-mimicking stem cell delivery via in situ coating of cells with a bioactive hyperbranched polyglycerol. J. Am. Chem. Soc. 2013, 135, 8770–8773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Chen, K.; Lee, H.-Y.; Xu, C.; Hsu, A.R.; Peng, S.; Chen, X.; Sun, S. Ultrasmall c (RGDyK)-Coated Fe3O4 nanoparticles and their specific targeting to integrin αvβ3-rich tumor cells. J. Am. Chem. Soc. 2008, 130, 7542–7543. [Google Scholar] [CrossRef] [Green Version]
- Qiao, R.; Liu, C.; Liu, M.; Hu, H.; Liu, C.; Hou, Y.; Wu, K.; Lin, Y.; Liang, J.; Gao, M. Ultrasensitive in Vivo detection of primary gastric tumor and lymphatic metastasis using upconversion nanoparticles. ACS Nano 2015, 9, 2120–2129. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, C.; Yin, X.; Cao, X.; Cai, M.; Xian, Y. Near-infrared fluorescent Ag2S nanodot-based signal amplification for efficient detection of circulating tumor cells. Anal. Chem. 2018, 90, 6702–6709. [Google Scholar] [CrossRef] [PubMed]
- Abbina, S.; Vappala, S.; Kumar, P.; Siren, E.M.J.; La, C.C.; Abbasi, U.; Brooks, D.E.; Kizhakkedathu, J.N. Hyperbranched polyglycerols: Recent advances in synthesis, biocompatibility and biomedical applications. J. Mater. Chem. B 2017, 5, 9249–9277. [Google Scholar] [CrossRef]
- Kainthan, R.K.; Hester, S.R.; Levin, E.; Devine, D.V.; Brooks, D.E. In vitro biological evaluation of high molecular weight hyperbranched polyglycerols. Biomaterials 2007, 28, 4581–4590. [Google Scholar] [CrossRef] [PubMed]
- Rossi, N.A.; Constantinescu, I.; Kainthan, R.K.; Brooks, D.E.; Scott, M.D.; Kizhakkedathu, J.N. Red blood cell membrane grafting of multi-functional hyperbranched polyglycerols. Biomaterials 2010, 31, 4167–4178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; He, B.; Huang, J.; Cheng, Z.; Xu, X.; Wei, C. Multihydroxy dendritic upconversion nanoparticles with enhanced water dispersibility and surface functionality for bioimaging. ACS Appl. Mater. Interfaces 2014, 6, 7719–7727. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Su, D.; Zeng, L.; Liu, N.; Jiang, L.; Feng, X.; Neoh, K.G.; Kang, E.-T. One-pot reaction for the large-scale synthesis of hyperbranched polyglycerol-grafted Fe3O4 nanoparticles. Dalton Trans. 2013, 42, 13642–13648. [Google Scholar] [CrossRef] [PubMed]
- Panja, P.; Das, P.; Mandal, K.; Jana, N.R. Hyperbranched polyglycerol grafting on the surface of silica-coated nanoparticles for high colloidal stability and low nonspecific interaction. ACS Sustain. Chem. Eng. 2017, 5, 4879–4889. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Z. Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials 2011, 32, 1110–1120. [Google Scholar] [CrossRef]
- Li, F.; Du, Y.; Liu, J.; Sun, H.; Wang, J.; Li, R.; Kim, D.; Hyeon, T.; Ling, D. Responsive assembly of upconversion nanoparticles for pH-activated and near-infrared-triggered photodynamic therapy of deep tumors. Adv. Mater. 2018, 30, e1802808. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.; Li, C.; He, F.; Hou, Z.; Huang, S.; Zhu, H.; Chen, X.; Lin, J. Poly(acrylic acid) modification of Nd3+-sensitized upconversion nanophosphors for highly efficient UCL imaging and pH-responsive drug delivery. Adv. Funct. Mater. 2015, 25, 4717–4729. [Google Scholar] [CrossRef]
- Dong, W.; Zhou, Y.; Yan, D.; Li, H.; Liu, Y. pH-responsive self-assembly of carboxyl-terminated hyperbranched polymers. Phys. Chem. Chem. Phys. 2007, 9, 1255. [Google Scholar] [CrossRef]
- Koç, F.; Wyszogrodzka, M.; Eilbracht, P.; Haag, R. Highly regioselective synthesis of amino-functionalized dendritic polyglycerols by a one-pot hydroformylation/reductive amination sequence. J. Org. Chem. 2005, 70, 2021–2025. [Google Scholar] [CrossRef]
- Moore, E.; Delalat, B.; Vasani, R.; Thissen, H.; Voelcker, N.H. Patterning and biofunctionalization of antifouling hyperbranched polyglycerol coatings. Biomacromolecules 2014, 15, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Delalat, B.; Vasani, R.; McPhee, G.; Thissen, H.; Voelcker, N.H. Surface-initiated hyperbranched polyglycerol as an ultralow-fouling coating on glass, silicon, and porous silicon substrates. ACS Appl. Mater. Interfaces 2014, 6, 15243–15252. [Google Scholar] [CrossRef]
- Kleifeld, O.; Doucet, A.; Keller, U.A.D.; Prudova, A.; Schilling, O.; Kainthan, R.K.; Starr, A.E.; Foster, L.J.; Kizhakkedathu, J.N.; Overall, C.M. Isotopic labeling of terminal amines in complex samples identifies protein N-termini and protease cleavage products. Nat. Biotechnol. 2010, 28, 281–288. [Google Scholar] [CrossRef]
- Kumar, S.; Aaron, J.; Sokolov, K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nat. Protoc. 2008, 3, 314–320. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, Y.; Meng, F.; Deng, C.; Cheng, R.; Zhang, J.; Feijen, J.; Zhong, Z. Highly efficacious and specific anti-glioma chemotherapy by tandem nanomicelles co-functionalized with brain tumor-targeting and cell-penetrating peptides. J. Control. Release 2018, 278, 1–8. [Google Scholar] [CrossRef]
- Sunder, A.; Hanselmann, R.; Frey, A.H.; Mülhaupt, R. Controlled synthesis of hyperbranched polyglycerols by ring-opening multibranching polymerization. Macromolecules 1999, 32, 4240–4246. [Google Scholar] [CrossRef]
- Wu, H.; Yin, T.; Li, K.; Wang, R.; Chen, Y.; Jing, L. Encapsulation property of hyperbranched polyglycerols as prospective drug delivery systems. Polym. Chem. 2018, 9, 300–306. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, M.; Wu, T.; Chen, Q.; Lian, X.; Wu, H.; Shi, B. Hyperbranched Polyglycerols as Robust Up-Conversion Nanoparticle Coating Layer for Feasible Cell Imaging. Polymers 2020, 12, 2592. https://doi.org/10.3390/polym12112592
Hao M, Wu T, Chen Q, Lian X, Wu H, Shi B. Hyperbranched Polyglycerols as Robust Up-Conversion Nanoparticle Coating Layer for Feasible Cell Imaging. Polymers. 2020; 12(11):2592. https://doi.org/10.3390/polym12112592
Chicago/Turabian StyleHao, Mingcong, Tongtong Wu, Qunzhi Chen, Xueyan Lian, Haigang Wu, and Bingyang Shi. 2020. "Hyperbranched Polyglycerols as Robust Up-Conversion Nanoparticle Coating Layer for Feasible Cell Imaging" Polymers 12, no. 11: 2592. https://doi.org/10.3390/polym12112592