A Non-Enzymatic Sensor Based on Fc-CHIT/CNT@Cu Nanohybrids for Electrochemical Detection of Glucose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
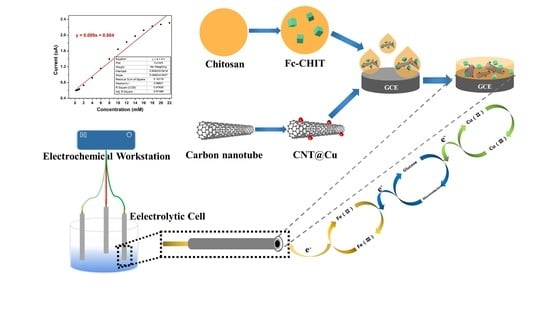
2.3. Synthesis of Ferrocene-Branched Chitosan
2.4. Decoration of Cu NPs onto Carbon Nanotubes (CNT@Cu)
2.5. Preparation of the Modified Electrodes
3. Results
3.1. Characterization of Composites
3.2. Electrochemical Measurement
3.2.1. Electrochemical Characterization of Modified Electrode
3.2.2. Electrochemical Activity of Fc-CHIT/CNT@Cu towards Glucose Detection
3.2.3. Selectivity and Stability of the Biosensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Fernandes, J.D.R.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pr. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ning, W.; Jia, S.; Shao, Y. Detection of glucose based on bimetallic PtCu nanochains modified electrodes. Anal. Chem. 2013, 85, 5040–5046. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, X.; Zhang, L.; Fan, J.; Wu, W. Construction of near-infrared photonic crystal glucose-sensing materials for ratiometric sensing of glucose in tears. Biosens. Bioelectron. 2013, 48, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Dhar, S.; Deka, N.; Debnath, K.; Mondal, S.P. Non-enzymatic salivary glucose detection using porous CuO nanostructures. Sens. Actuators B Chem. 2020, 302, 127134. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Hajari, N.; Turner, A.P.; Tiwari, A. A high-performance glucose biosensor using covalently immobilised glucose oxidase on a poly(2,6-diaminopyridine)/carbon nanotube electrode. Talanta 2013, 116, 801–808. [Google Scholar] [CrossRef]
- Matysiak-Brynda, E.; Sęk, J.P.; Kasprzak, A.; Królikowska, A.; Donten, M.; Patrzalek, M.; Poplawska, M.; Nowicka, A.M. Reduced graphene oxide doping with nanometer-sized ferrocene moieties–New active material for glucose redox sensors. Biosens. Bioelectron. 2019, 128, 23–31. [Google Scholar] [CrossRef]
- Li, H.; Zhao, F.; Yue, L.; Li, S.; Xiao, F. Nonenzymatic electrochemical biosensor based on novel hydrophilic ferrocene-terminated hyperbranched polymer and its application in glucose detection. Electroanalysis 2015, 28, 1003–1011. [Google Scholar] [CrossRef]
- Grochowska, K.; Ryl, J.; Karczewski, J.; Śliwiński, G.; Cenian, A.; Siuzdak, K. Non-enzymatic flexible glucose sensing platform based on nanostructured TiO2—Au composite. J. Electroanal. Chem. 2019, 837, 230–239. [Google Scholar] [CrossRef]
- Tabassum, S.; Naz, S.; Nisar, A.; Sun, H.; Karim, S.; Khan, M.; Shahzada, S.; Rahman, A.U.; Ahmad, M.; Tabussam, S.; et al. Synergic effect of plasmonic gold nanoparticles and graphene oxide on the performance of glucose sensing. New J. Chem. 2019, 43, 18925–18934. [Google Scholar] [CrossRef]
- Gao, W.; Li, Q.; Dou, M.; Zhang, Z.; Wang, F. Self-supported Ni nanoparticles embedded on nitrogen-doped carbon derived from nickel polyphthalocyanine for high-performance non-enzymatic glucose detection. J. Mater. Chem. B 2018, 6, 6781–6787. [Google Scholar] [CrossRef]
- Mei, H.; Wu, W.; Yu, B.; Li, Y.; Wu, H.; Wang, S.; Xia, Q. Non-enzymatic sensing of glucose at neutral pH values using a glassy carbon electrode modified with carbon supported Co@Pt core-shell nanoparticles. Microchim. Acta 2015, 182, 1869–1875. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, P.; Liu, Y.; Luo, H. A novel non-enzymatic glucose sensor based on a Cu-nanoparticle-modified graphene edge nanoelectrode. Chem. Sci. 2017, 9, 2205–2210. [Google Scholar] [CrossRef]
- Prakash, S.; Chakrabarty, T.; Singh, A.K.; Shahi, V.K. Polymer thin films embedded with metal nanoparticles for electrochemical biosensors applications. Biosens. Bioelectron. 2013, 41, 43–53. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, L.; Peng, C.; Su, Y.; Yang, Z.; Zhang, L.; Wei, H.; Zhang, Y. A non-enzymatic glucose sensor based on the composite of cubic Cu nanoparticles and arc-synthesized multi-walled carbon nanotubes. Biosens. Bioelectron. 2013, 47, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.S.; Le, Q.H.; Yoshikawa, H.; Saito, M.; Tamiya, E. Development of non-enzymatic electrochemical glucose sensor based on graphene oxide nanoribbon–gold nanoparticle hybrid. Electrochim. Acta 2014, 146, 98–105. [Google Scholar] [CrossRef]
- Banks, C.E.; Crossley, A.; Salter, C.; Wilkins, S.J.; Compton, R.G. Carbon nanotubes contain metal impurities which are responsible for the “electrocatalysis” seen at some nanotube-modified electrodes. Angew. Chem. Int. Ed. Engl. 2006, 45, 2533–2537. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, Y.; Gong, Q.; Xia, Z.; Yang, Y.; Liu, X.; Wang, J.; Gao, Q. Polythiophene grafted onto single-wall carbon nanotubes through oligo(ethylene oxide) linkages for supercapacitor devices with enhanced electrochemical performance. ChemElectroChem 2019, 6, 4595–4607. [Google Scholar] [CrossRef]
- Başkaya, G.; Yıldız, Y.; Savk, A.; Okyay, T.O.; ERiŞ, S.; Sert, H.; Şen, F. Rapid, sensitive, and reusable detection of glucose by highly monodisperse nickel nanoparticles decorated functionalized multi-walled carbon nanotubes. Biosens. Bioelectron. 2017, 91, 728–733. [Google Scholar] [CrossRef]
- Sun, Y.P.; Fu, K.; Lin, Y.; Huang, W. Functionalized carbon nanotubes: Properties and applications. Acc. Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef]
- Zanella, R.; Basiuk, V.A.; Santiago, P.; Basiuk, V.A.; Mireles, E.; Puente-Lee, I.; Saniger, J.M. Deposition of gold nanoparticles onto thiol-functionalized multiwalled carbon nanotubes. J. Phys. Chem. B 2005, 109, 16290–16295. [Google Scholar] [CrossRef]
- Miao, C.; Zhang, A.; Xu, Y.; Chen, S.; Ma, F.; Huang, C.; Jia, N.-Q. An ultrasensitive electrochemiluminescence sensor for detecting diphenhydramine hydrochloride based on l-cysteine-functionalized multiwalled carbon nanotubes/gold nanoparticles nanocomposites. Sens. Actuators B Chem. 2015, 213, 5–11. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, Y.; Yin, X.; Yang, H.; Cui, J.; Zhu, T.; Li, G. Polydopamine-based photonic crystal structures. J. Mater. Chem. C 2013, 1, 6136. [Google Scholar] [CrossRef]
- Kavosi, B.; Salimi, A.; Hallaj, R.; Amani, K. A highly sensitive prostate-specific antigen immunosensor based on gold nanoparticles/PAMAM dendrimer loaded on MWCNTS/chitosan/ionic liquid nanocomposite. Biosens. Bioelectron. 2014, 52, 20–28. [Google Scholar] [CrossRef]
- Kalita, G.; Sharma, S.; Wakita, K.; Umeno, M.; Hayashi, Y.; Tanemura, M. A photoinduced charge transfer composite of graphene oxide and ferrocene. Phys. Chem. Chem. Phys. 2013, 15, 1271–1274. [Google Scholar] [CrossRef]
- Tan, G.; Qiu, Y.; Qiu, Y.; Huang, W.; Fan, H.; Ren, B. Supercapacitors based on polyelectrolyte/ferrocenyl-surfactant complexes with high rate capability. RSC Adv. 2016, 6, 31632–31638. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Lin, J.T.; Yang, H. Ferrocene-modified chitosan as an efficient and green heterogeneous catalyst for sulfate-radical-based advanced oxidation process. Carbohydr. Polym. 2017, 173, 412–421. [Google Scholar] [CrossRef]
- Jha, N.; Ramaprabhu, S. Synthesis and thermal conductivity of copper nanoparticle decorated multiwalled carbon nanotubes based nanofluids. J. Phys. Chem. C 2008, 112, 9315–9319. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Li, Y.K.; Wang, C.Q. Oxidation and pH responsive nanoparticles based on ferrocene-modified chitosan oligosaccharide for 5-fluorouracil delivery. Carbohydr. Polym. 2014, 114, 27–35. [Google Scholar] [CrossRef]
- Rabti, A.; Mayorga-Martinez, C.C.; Baptista-Pires, L.; Raouafi, N.; Merkoçi, A. Ferrocene-functionalized graphene electrode for biosensing applications. Anal. Chim. Acta 2016, 926, 28–35. [Google Scholar] [CrossRef]
- Ma, P.; Xiaoyan, M.; Suo, Q.; Chen, F. Cu NPs@NiF electrode preparation by rapid one-step electrodeposition and its sensing performance for glucose. Sens. Actuators B Chem. 2019, 292, 203–209. [Google Scholar] [CrossRef]
- Zhang, C.; Li, F.; Huang, S.; Li, M.; Guo, T.; Mo, C.; Pang, X.; Chen, L.; Li, X. In-situ facile preparation of highly efficient copper/nickel bimetallic nanocatalyst on chemically grafted carbon nanotubes for nonenzymatic sensing of glucose. J. Colloid Interface Sci. 2019, 557, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Khosroshahia, Z.; Karimzadeh, F.; Kharazihaa, M.; Allafchianb, A. A non-enzymatic sensor based on three-dimensional graphene foam decorated with Cu-xCu2O nanoparticles for electrochemical detection of glucose and its application in human serum. Mater. Sci. Eng. C 2020, 108, 110216. [Google Scholar] [CrossRef] [PubMed]
- Teimuri-Mofrad, R.; Aghaiepour, A.; Rahimpour, K. A convenient method for synthesis of novel alkylferrocene derivatives with various functional groups: Synthesis, characterization and electrochemical investigation. J. Iran. Chem. Soc. 2020, 17, 2449–2462. [Google Scholar] [CrossRef]
- Jiang, Z.; Shangguan, Y.; Zheng, Q. Ferrocene-modified polyelectrolyte film-coated electrode and its application in glucose detection. Polymers 2019, 11, 551. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Wang, Y.; Wang, H.; Hou, S. Gold nanoparticles decorated on single layer graphene applied for electrochemical ultrasensitive glucose biosensor. J. Electroanal. Chem. 2019, 855, 113495. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Jafari-Asl, M.; Dorostkar, N.; Ghiaci, M.; Martínez-Huerta, M.V.; Fierro, J.L.G. The fabrication and characterization of Cu-nanoparticle immobilization on a hybrid chitosan derivative-carbon support as a novel electrochemical sensor: Application for the sensitive enzymeless oxidation of glucose and reduction of hydrogen peroxide. J. Mater. Chem. B 2014, 2, 706–717. [Google Scholar] [CrossRef]
- Kangkamano, T.; Numnuam, A.; Limbut, W.; Kanatharana, P.; Thavarungkul, P. Chitosan cryogel with embedded gold nanoparticles decorated multiwalled carbon nanotubes modified electrode for highly sensitive flow based non-enzymatic glucose sensor. Sens. Actuators B Chem. 2017, 246, 854–863. [Google Scholar] [CrossRef]
- Sivasankar, K.; Rani, K.K.; Wang, S.F.; Devasenathipathy, R.; Lin, C.H. Copper nanoparticle and nitrogen doped graphite oxide based biosensor for the sensitive determination of glucose. Nanomaterials 2018, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- SoYoon, S.; Ramadoss, A.; Saravanakumar, B.; Kim, S.J. Novel Cu/CuO/ZnO hybrid hierarchical nanostructures for non-enzymatic glucose sensor application. J. Electroanal. Chem. 2014, 717-718, 90–95. [Google Scholar] [CrossRef]
- Liu, D.; Luo, Q.; Zhou, F. Nonenzymatic glucose sensor based on gold–copper alloy nanoparticles on defect sites of carbon nanotubes by spontaneous reduction. Synth. Met. 2010, 160, 1745–1748. [Google Scholar] [CrossRef]
- Male, K.B.; Hrapovic, S.; Liu, Y.; Wang, D.; Luong, J.H. Electrochemical detection of carbohydrates using copper nanoparticles and carbon nanotubes. Analytica Chimica Acta 2004, 516, 35–41. [Google Scholar] [CrossRef]
- Gupta, J.; Arya, S.; Verma, S.; Singh, A.; Sharma, A.; Singh, B.; Prerna; Sharma, R. Performance of template-assisted electrodeposited Copper/Cobalt bilayered nanowires as an efficient glucose and Uric acid senor. Mater. Chem. Phys. 2019, 238, 121969. [Google Scholar] [CrossRef]
- Sattayasamitsathit, S.; Thavarungkul, P.; Thammakhet, C.; Limbut, W.; Numnuam, A.; Buranachai, C.; Kanatharana, P. Fabrication of nanoporous copper film for electrochemical detection of glucose. Electroanalysis 2009, 21, 2371–2377. [Google Scholar] [CrossRef]
- Karikalan, N.; Karthik, R.; Chen, S.M.; Karuppiah, C.; Elangovan, A. Sonochemical synthesis of sulfur doped reduced graphene oxide supported CuS nanoparticles for the non-enzymatic glucose sensor applications. Sci. Rep. 2017, 7, 2494. [Google Scholar] [CrossRef]
- Yang, P.; Wang, X.; Ge, C.Y.; Fu, X.; Liu, X.Y.; Chai, H.; Guo, X.; Yao, H.C.; Xu, C.; Chen, K. Fabrication of CuO nanosheets-built microtubes via Kirkendall effect for non-enzymatic glucose sensor. Appl. Surf. Sci. 2019, 494, 484–491. [Google Scholar] [CrossRef]
- Figiela, M.; Wysokowski, M.; Galiński, M.; Jesionowski, T.; Stepniak, I. Synthesis and characterization of novel copper oxide-chitosan nanocomposites for non-enzymatic glucose sensing. Sens. Actuators B Chem. 2018, 272, 296–307. [Google Scholar] [CrossRef]
- Lu, L.; Kang, J. Amperometric nonenzymatic sensing of glucose at very low working potential by using a nanoporous PdAuNi ternary alloy. Microchim. Acta 2018, 185, 111. [Google Scholar] [CrossRef]







| Modified Electrode | LOD (μM) | Linear Range (mM) | Sensitivity (μAmM−1cm−2) | Electrolyte pH | Ref. |
|---|---|---|---|---|---|
| Cu@CHIT–CNT | 5 × 10−2 | 0.5 × 10−3~1 | Not mentioned | 12.7 | [36] |
| AuNPs-MWCNTs-CHIT cryogel | 0.5 | 1 × 10−3~1 | Not mentioned | 12.7 | [37] |
| Cu/NiNPs/CMWCNTs-ITO | 0.67 | 1 × 10−3~1 | 6.782 | 13 | [31] |
| rGO-Fc/GA-GOx/GCE | 2 × 10−2 | 2~10 × 10−3 | Not mentioned | 7.4 | [6] |
| CuNPs/NGO | 0.44 | 0.001~1.803 | 2500 | 13 | [38] |
| Cu/CuO/ZnO | 18 | 0.1~1 | 408 | 13 | [39] |
| AuCu/CNTs | 4 | 0.08~9.260 | 22 | 13 | [40] |
| Cu NPs/SWCNTs | 0.3 | 0.5~500 × 10−3 | 0.256 | 12.3 | [41] |
| Cu/Co NWs | 5 × 10−2 | 0.3~2.6 × 10−3 | 0.097 | 13.7 | [42] |
| Cu nanoporous | 40 | 0.01~0.5 | 220 | 13.3 | [43] |
| S-rGO/CuS | 0.032 | up to 20.17 | 429.4 | 13 | [44] |
| CuO-6 | 0.307 | up to 5.664 | 992.073 | 12.7 | [45] |
| CuO-CS/GCE | 11 | 0.05~1 | 503.129 | 13 | [46] |
| Fc-CHIT/CNT@Cu/GCE | 13.52 | 0.2~22 | 1.256 | 13 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Hu, S.; Shi, F.; Huang, K.; Li, J. A Non-Enzymatic Sensor Based on Fc-CHIT/CNT@Cu Nanohybrids for Electrochemical Detection of Glucose. Polymers 2020, 12, 2419. https://doi.org/10.3390/polym12102419
Wang F, Hu S, Shi F, Huang K, Li J. A Non-Enzymatic Sensor Based on Fc-CHIT/CNT@Cu Nanohybrids for Electrochemical Detection of Glucose. Polymers. 2020; 12(10):2419. https://doi.org/10.3390/polym12102419
Chicago/Turabian StyleWang, Fang, Sheng Hu, Fengna Shi, Kexin Huang, and Jiarui Li. 2020. "A Non-Enzymatic Sensor Based on Fc-CHIT/CNT@Cu Nanohybrids for Electrochemical Detection of Glucose" Polymers 12, no. 10: 2419. https://doi.org/10.3390/polym12102419