Proper Blends of Biodegradable Polycaprolactone and Natural Rubber for 3D Printing
Abstract
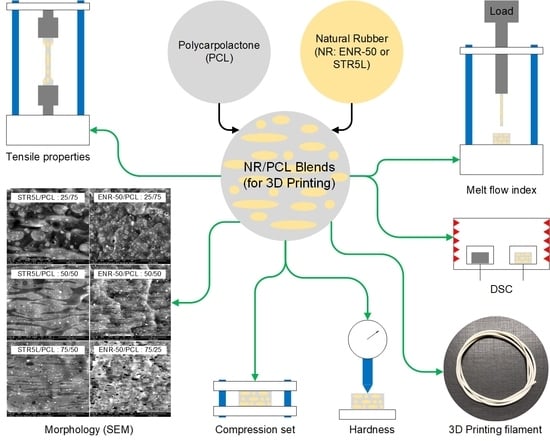
:1. Introduction
2. Materials and Methods
2.1. Compounding Preparation
2.2. Surface Morphology
2.3. Thermal Property Testing
2.4. Melt Flow Index (MFI) Testing
2.5. Compression Set Testing
2.6. Hardness Testing
2.7. Tensile Testing
3. Results and Discussion
3.1. Surface Morphology
3.2. Melting Properties
3.3. Compression Set
3.4. Hardness
3.5. Tensile Testing
3.6. Preliminary Filament Extrusion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, J.-Y.; An, J.; Chua, C.K. Fundamentals and applications of 3D printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Ploch, C.C.; Mansi, C.S.; Jayamohan, J.; Kuhl, E. Using 3D Printing to Create Personalized Brain Models for Neurosurgical Training and Preoperative Planning. World Neurosurg. 2016, 90, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Konta, A.A.; García-Piña, M.; Serrano, D. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, F.N.; Iovenitti, P.; Masood, S.H.; Nikzad, M. In-plane energy absorption evaluation of 3D printed polymeric honeycombs. Virtual Phys. Prototyp. 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Raj, S.A.; Muthukumaran, E.; Jayakrishna, K. A Case Study of 3D Printed PLA and Its Mechanical Properties. In Proceedings of the 4th International Conference on Mechanical, Materials and Manufacturing (ICMMM 2017), Atlanta, GA, USA, 25–27 October 2017; pp. 11219–11226. [Google Scholar]
- Ferreira, R.T.L.; Amatte, I.C.; Dutra, T.A.; Bürger, D. Experimental characterization and micrography of 3D printed PLA and PLA reinforced with short carbon fibers. Compos. Part B 2017, 124, 88–100. [Google Scholar] [CrossRef]
- Torrado Perez, A.R.; Roberson, D.A.; Wicker, R.B. Fracture Surface Analysis of 3D-Printed Tensile Specimens of Novel ABS-Based Materials. J. Fail. Anal. Prev. 2014, 14, 343–353. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Song, W.; Yee, K.; Lee, K.-Y.; Tagarielli, V. Measurements of the mechanical response of unidirectional 3D-printed PLA. Mater. Des. 2017, 123, 154–164. [Google Scholar] [CrossRef]
- Letcher, T.; Waytashek, M. Material Property Testing of 3D-Printed Specimen in PLA on an Entry-Level 3D Printer. In Proceedings of the ASME 2014 International Mechanical Engineering Congress & Exposition (IMECE 2014), Montreal, QC, Canada, 14–20 November 2014; pp. 1–14. [Google Scholar]
- Dul, S.; Fambri, L.; Pegoretti, A. Filaments Production and Fused Deposition Modelling of ABS/Carbon Nanotubes Composites. Nanomaterials 2018, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Isa, N.M.A.; Sa’Ude, N.; Ibrahim, M.; Hamid, S.M.; Kamarudin, K. A Study on Melt Flow Index on Copper-ABS for Fused Deposition Modeling (FDM) Feedstock. Appl. Mech. Mater. 2015, 773, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Liu, T.; Yang, C.; Wang, Q.; Li, D. Interface and performance of 3D printed continuous carbon fiber reinforced PLA composites. Compos. Part A 2016, 88, 198–205. [Google Scholar] [CrossRef]
- Liu, W.; Wu, N.; Pochiraju, K.V. Shape recovery characteristics of SiC/C/PLA composite filaments and 3D printed parts. Compos. Part A 2018, 108, 1–11. [Google Scholar] [CrossRef]
- Pongtanayut, K.; Thongpin, C.; Santawitee, O. The Effect of Rubber on Morphology, Thermal Properties and Mechanical Properties of PLA/NR and PLA/ENR Blends. In Proceedings of the 10th Eco-Energy and Materials Science and Engineering (EMSES2012), Ubon Ratchathani, Thailand, 5–8 December 2012; pp. 888–897. [Google Scholar]
- Nakason, C.; Wannavilai, P.; Kaesaman, A. Thermoplastic vulcanizates based on epoxidized natural rubber/polypropylene blends: Effect of epoxide levels in ENR molecules. J. Appl. Polym. Sci. 2006, 101, 3046–3052. [Google Scholar] [CrossRef]
- Masek, A.; Zaborski, M. ENR/PCL Polymer biocomposites from renewable resources. Comptes Rendus Chim. 2014, 17, 944–951. [Google Scholar] [CrossRef]
- Masek, A.; Zaborski, M.; Piotrowska, M. Controlled degradation of biocomposites ENR/PCL containing natural antioxidants. Comptes Rendus Chim. 2014, 17, 1128–1135. [Google Scholar] [CrossRef]
- Yunus, W.M.Z.W.; Siong, L.C.; Manroshan, S.; Hussein, M.Z.; Ab Rahman, M.Z.; Dahlan, K.Z.M. Preparation and Characterisation of Crosslinked Polycaprolactone and Natural Rubber (SMR CV60) Blends. J. Rubber Res. 2013, 16, 147–161. Available online: http://vitaldoc.lgm.gov.my:8060/vital/access/services/Download/vital1:85529/ATTACHMENT01 (accessed on 17 October 2020).
- Formela, K.; Marć, M.; Namieśnik, J.; Zabiegała, B. The estimation of total volatile organic compounds emissions generated from peroxide-cured natural rubber/polycaprolactone blends. Microchem. J. 2016, 127, 30–35. [Google Scholar] [CrossRef]
- Formela, K.; Marć, M.; Wang, S.; Saeb, M.R. Interrelationship between total volatile organic compounds emissions, structure and properties of natural rubber/polycaprolactone bio-blends cross-linked with peroxides. Polym. Test. 2017, 60, 405–412. [Google Scholar] [CrossRef]
- Mishra, J.K.; Chang, Y.-W.; Kim, D.-K. Green thermoplastic elastomer based on polycaprolactone/epoxidized natural rubber blend as a heat shrinkable material. Mater. Lett. 2007, 61, 3551–3554. [Google Scholar] [CrossRef]
- Mishra, J.K.; Chang, Y.-W.; Kim, W. The effect of peroxide crosslinking on thermal, mechanical, and rheological properties of polycaprolactone/epoxidized natural rubber blends. Polym. Bull. 2010, 66, 673–681. [Google Scholar] [CrossRef]
- Faibunchan, P.; Pichaiyut, S.; Chueangchayaphan, W.; Kummerlöwe, C.; Venneman, N.; Nakason, C. Influence type of natural rubber on properties of green biodegradable thermoplastic natural rubber based on poly(butylene succinate). Polym. Adv. Technol. 2019, 30, 1010–1026. [Google Scholar] [CrossRef]










| Sample Code | Sample Name | Quantity (w/w) | Quantity (phr) | ||||
|---|---|---|---|---|---|---|---|
| STR5L | ENR-50 | PCL | ZnO | Wingstay L | Paraffinic Oil | ||
| A | STR5L/PCL 40/60 | 40 | 60 | 5 | 1 | 20 | |
| B | STR5L/PCL 50/50 | 50 | 50 | 5 | 1 | 20 | |
| C | STR5L/PCL 60/40 | 60 | 40 | 5 | 1 | 20 | |
| D | ENR-50/PCL 40/60 | 40 | 60 | 5 | 1 | 20 | |
| E | ENR-50/PCL 50/50 | 50 | 50 | 5 | 1 | 20 | |
| F | ENR-50/PCL 60/40 | 60 | 40 | 5 | 1 | 20 | |
| Step | Time (min) | Component Adding |
|---|---|---|
| 1 | 5 | Rubber (STR5L or ENR-50) |
| 2 | 2 | Zinc oxide |
| 3 | 2 | Wingstay L |
| 4 | 5 | Paraffinic oil |
| 5 | - | Unloading rubber compound |
| 6 | 5 | PCL |
| 7 | 5 | ENR-50 or STR5L compound |
| Samples | Tg1 (°C) | Tg2 (°C) | Tm (°C) | ∆H (J/g) |
|---|---|---|---|---|
| PCL | −53.47 | - | 50.9 | 51.8 |
| A | −72.01 | −27.87 | 50.6 | 13.6 |
| B | −72.92 | - | 49.5 | 11.2 |
| C | −71.72 | −39.29 | 49.0 | 17.9 |
| D | −29.81 | −9.45 | 49.9 | 37.6 |
| E | −32.77 | −10.27 | 48.4 | 18.0 |
| F | −30.55 | −14.93 | 49.1 | 22.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wissamitanan, T.; Dechwayukul, C.; Kalkornsurapranee, E.; Thongruang, W. Proper Blends of Biodegradable Polycaprolactone and Natural Rubber for 3D Printing. Polymers 2020, 12, 2416. https://doi.org/10.3390/polym12102416
Wissamitanan T, Dechwayukul C, Kalkornsurapranee E, Thongruang W. Proper Blends of Biodegradable Polycaprolactone and Natural Rubber for 3D Printing. Polymers. 2020; 12(10):2416. https://doi.org/10.3390/polym12102416
Chicago/Turabian StyleWissamitanan, Thossapit, Charoenyutr Dechwayukul, Ekwipoo Kalkornsurapranee, and Wiriya Thongruang. 2020. "Proper Blends of Biodegradable Polycaprolactone and Natural Rubber for 3D Printing" Polymers 12, no. 10: 2416. https://doi.org/10.3390/polym12102416