Synthesis and Physicochemical Characterization of Undecylenic Acid Grafted to Hyaluronan for Encapsulation of Antioxidants and Chemical Crosslinking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
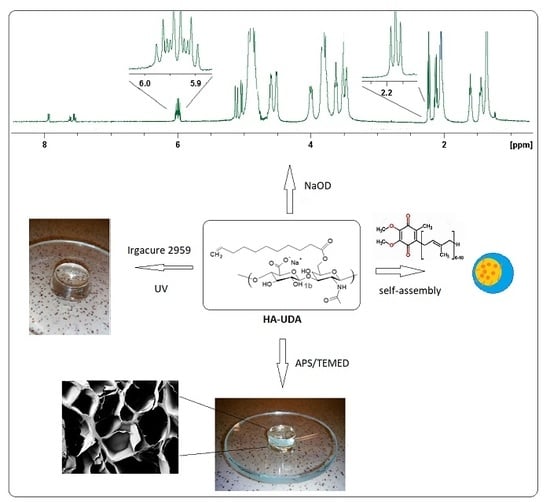
2.2. General Procedure for the Synthesis of Undecenoyl-Hyaluronan
2.3. Nuclear Magnetic Resonance Spectroscopy
2.4. Determination of Degree of Substitution of the Degree of Substitution by Gas Chromatography (GC)
2.5. Infrared Spectroscopy
2.6. Determination of Molecular Weight (Mw) by Size-Exclusion Chromatography (SEC)-Multiangle Laser Scattering (MALLS)
2.7. In Vitro Cell Compatibility of the Derivative HA-UDA
2.8. Preparation of Polymeric Micelles by Solvent Evaporation
2.9. Dynamic Light Scattering (DLS) Analysis
2.10. Rheological Characterization
2.11. Radical Polymerization Mediated by APS/TEMED
2.12. Photo-Polymerization Mediated by Irgacure
2.13. Scanning Electron Microscopy (SEM)
2.14. Determination of Mass Swelling Ratio (Qm)
3. Results and Discussion
3.1. Chemical Modification of Hyaluronic Acid Mediated by Mixed Anhydrides
3.2. Structural Characterization of the Conjugate HA-UDA
3.3. Rheological Characterization
3.4. Drug Loading in HA-UDA
3.5. Determination of Cell Viability after Chemical Modification
3.6. Crosslinking of HA-UDA
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Son, S.U.; Lim, J.W.; Kang, T.; Jung, J.; Lim, E.K. Hyaluronan-based nanohydrogels as effective carriers for transdermal delivery of lipophilic agents: Towards transdermal drug administration in neurological disorders. Nanomaterials 2017, 7, 427. [Google Scholar] [CrossRef] [Green Version]
- Antunes, J.; Gaspar, V.M.; Ferreira, L.; Monteiro, M.; Henrique, R.; Jerónimo, C.; Mano, J.F. In-air production of 3D co-culture tumor spheroid hydrogels for expedited drug screening. Acta Biomater. 2019, 94, 392–409. [Google Scholar] [CrossRef]
- Pekař, M. Hydrogels with Micellar Hydrophobic (Nano)Domains. Front. Mater. 2015, 1, 35. [Google Scholar] [CrossRef] [Green Version]
- Okay, O. Semicrystalline physical hydrogels with shape-memory and self-healing properties. J. Mater. Chem. B 2019, 7, 1581–1596. [Google Scholar] [CrossRef]
- Burdick, J.A.; Chung, C.; Jia, X.; Randolph, M.A.; Langer, R. Controlled Degradation and Mechanical Behavior of Photopolymerized Hyaluronic Acid Networks. Biomacromolecules 2005, 6, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Bencherif, S.A.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials 2008, 29, 1739–1749. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Goversen, B.; de Ruijter, M.; Abbadessa, A.; Melchels, F.P.W.; Oner, F.C.; Dhert, W.J.; Vermonden, T.; Alblas, J. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef] [Green Version]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Cho, H.J. Mitochondria Targeting and Destabilizing Hyaluronic Acid Derivative-Based Nanoparticles for the Delivery of Lapatinib to Triple-Negative Breast Cancer. Biomacromolecules 2019, 20, 835–845. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, N.; Gong, X.; Kawashima, Y.; Cui, F.; Sun, S. Tumor-targeting micelles based on folic acid and alpha-tocopherol succinate conjugated hyaluronic acid for paclitaxel delivery. Colloids Surf. B Biointerfaces 2019, 177, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Achbergerová, E.; Šmejkalová, D.; Huerta-Angeles, G.; Souček, K.; Hermannová, M.; Vágnerová, H.; Vícha, R.; Velebný, V. In vivo monitoring of tumor distribution of hyaluronan polymeric micelles labeled or loaded with near-infrared fluorescence dye. Carbohydr. Polym. 2018, 198, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Bongiovì, F.; Fiorica, C.; Palumbo, F.S.; Di Prima, G.; Giammona, G.; Pitarresi, G. Imatinib-Loaded Micelles of Hyaluronic Acid Derivatives for Potential Treatment of Neovascular Ocular Diseases. Mol. Pharm. 2018, 15, 5031–5045. [Google Scholar] [CrossRef] [PubMed]
- Šmejkalová, D.; Muthný, T.; Nešporová, K.; Hermannová, M.; Achbergerová, E.; Huerta-Angeles, G.; Svoboda, M.; Čepa, M.; Machalová, V.; Luptáková, D.; et al. Hyaluronan polymeric micelles for topical drug delivery. Carbohydr. Polym. 2017, 156, 86–96. [Google Scholar] [CrossRef]
- Huerta-Angeles, G.; Brandejsová, M.; Kulhánek, J.; Pavlík, V.; Šmejkalová, D.; Vágnerová, H.; Velebný, V. Linolenic acid grafted hyaluronan: Process development, structural characterization, biological assessing, and stability studies. Carbohydr. Polym. 2016, 152, 815–824. [Google Scholar] [CrossRef]
- Šmejkalová, D.; Nešporova, K.; Huerta-Angeles, G.; Syrovatka, J.; Jirák, D.; Gálisová, A.; Velebny, V. Selective In Vitro Anticancer Effect of Superparamagnetic Iron Oxide Nanoparticles Loaded in Hyaluronan Polymeric Micelles. Biomacromolecules 2014, 15, 4012–4020. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; He, Q.; Wu, Y.; Lu, Z.; Sun, J.; Liu, Z.; Shao, Y.; Wang, A. Oleic acid induces apoptosis and autophagy in the treatment of Tongue Squamous cell carcinomas. Sci. Rep. 2017, 7, 11277. [Google Scholar] [CrossRef] [Green Version]
- Laskar, K.; Faisal, S.M.; Rauf, A.; Ahmed, A.; Owais, M. Undec-10-enoic acid functionalized chitosan based novel nano-conjugate: An enhanced anti-bacterial/biofilm and anti-cancer potential. Carbohydr. Polym. 2017, 166, 14–23. [Google Scholar] [CrossRef]
- Machado, T.O.; Cardoso, P.B.; Feuser, P.E.; Sayer, C.; Araújo, P.H.H. Thiol-ene miniemulsion polymerization of a biobased monomer for biomedical applications. Colloids Surf. B Biointerfaces 2017, 159, 509–517. [Google Scholar] [CrossRef]
- Meng, X.; Roy Choudhury, S.; Edgar, K.J. Multifunctional cellulose esters by olefin cross-metathesis and thiol-Michael addition. Polym. Chem. 2016, 7, 3848–3856. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Matson, J.B.; Edgar, K.J. Olefin Cross-Metathesis in Polymer and Polysaccharide Chemistry: A Review. Biomacromolecules 2017, 18, 1661–1676. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Chung, K.; Kim, D.Y.; Lee, S.H.; Kim, J.S.; Rhee, Y.H. Preparation and biocompatibility of crosslinked poly(3-hydroxyundecenoate). Int. J. Biol. Macromol. 2018, 107, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef] [PubMed]
- Vištejnová, L.; Dvořakova, J.; Hasová, M.; Muthný, T.; Velebný, V.; Souček, K.; Kubala, L. The comparison of impedance-based method of cell proliferation monitoring with commonly used metabolic-based techniques. Neuro Endocrinol. Lett. 2009, 30, 121–127. [Google Scholar] [PubMed]
- Matelová, A.; Huerta-Angeles, G.; Šmejkalová, D.; Brůnová, Z.; Dušek, J.; Vícha, R.; Velebný, V. Synthesis of novel amphiphilic hyaluronan containing-aromatic fatty acids for fabrication of polymeric micelles. Carbohydr. Polym. 2016, 151, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.M. Rheology of non-Newtonian fluids: A new flow equation for pseudoplastic systems. J. Colloid Sci. 1965, 20, 417–437. [Google Scholar] [CrossRef]
- Hiemstra, C.; Zhou, W.; Zhong, Z.; Wouters, M.; Feijen, J. Rapidly in Situ Forming Biodegradable Robust Hydrogels by Combining Stereocomplexation and Photopolymerization. J. Am. Chem. Soc. 2007, 129, 9918–9926. [Google Scholar] [CrossRef]
- Chmelar, J.; Kotzianova, A.; Hermannova, M.; Sulakova, R.; Smejkalova, D.; Kulhanek, J.; Velebný, V. Evaluating the degree of substitution of water-insoluble acyl derivatives of hyaluronan using Raman spectroscopy: Method development and comparison with gas chromatography and 1H NMR. Anal. Methods 2017, 9, 232–239. [Google Scholar] [CrossRef]
- Ret, D.; Steiner, G.; Gentilini, S.; Knaus, S. Exact determination of the degree of substitution of high molar mass hyaluronan by controlling the conformation in solution. Carbohydr. Polym. 2019, 204, 124–130. [Google Scholar] [CrossRef]
- Simon, S.; Dugast, J.Y.; Le Cerf, D.; Picton, L.; Muller, G. Amphiphilic polysaccharides. Evidence for a competition between intra and intermolecular associations in dilute system. Polymer 2003, 44, 7917–7924. [Google Scholar]
- Knott, A.; Achterberg, V.; Smuda, C.; Mielke, H.; Sperling, G.; Dunckelmann, K.; Vogelsang, A.; Krüger, A.; Schwengler, H.; Behtash, M.; et al. Topical treatment with coenzyme Q10-containing formulas improves skin’s Q10 level and provides antioxidative effects. Biofactors 2015, 41, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Camacho, J.D.; Bernier, M.; López-Lluch, G.; Navas, P. Coenzyme Q(10) Supplementation in Aging and Disease. Front. Physiol. 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrou, A.L.; Petrou, P.L.; Ntanos, T.; Liapis, A. A Possible Role for Singlet Oxygen in the Degradation of Various Antioxidants. A Meta-Analysis and Review of Literature Data. Antioxidants 2018, 7, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xia, Q.; Li, Y.; He, Z.; Li, Z.; Guo, T.; Wu, Z.; Feng, N. CD44 Assists the Topical Anti-Psoriatic Efficacy of Curcumin-Loaded Hyaluronan-Modified Ethosomes: A New Strategy for Clustering Drug in Inflammatory Skin. Theranostics 2019, 9, 48–64. [Google Scholar] [CrossRef]
- Aguirre, G.; Khoukh, A.; Taboada, P.; Chougrani, K.; Alard, V.; Billon, L. Smart self-assembled microgel films as encapsulating carriers for UV-absorbing molecules. Polym. Chem. 2018, 9, 1155–1159. [Google Scholar] [CrossRef]
- Le, L.V.; Mohindra, P.; Fang, Q.; Sievers, R.E.; Mkrtschjan, M.A.; Solis, C.; Safranek, C.W.; Russell, B.; Lee, R.J.; Desai, T.A. Injectable hyaluronic acid based microrods provide local micromechanical and biochemical cues to attenuate cardiac fibrosis after myocardial infarction. Biomaterials 2018, 169, 11–21. [Google Scholar] [CrossRef]
- Huerta-Ángeles, G.; Nešporová, K.; Ambrožová, G.; Kubala, L.; Velebný, V. An Effective Translation: The Development of Hyaluronan-Based Medical Products from the Physicochemical, and Preclinical Aspects. Front. Bioeng. Biotechnol. 2018, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.; Zhu, W.; Yu, L.; Ding, J. Negative cooperative effect of cytotoxicity of a di-component initiating system for a novel injectable tissue engineering hydrogel. Chin. Sci. Bull. 2005, 50, 1093–1096. [Google Scholar] [CrossRef]
- Char, C.; Padilla, C.; Campos, V.; Pepczynska, M.; Díaz-Calderón, P.; Enrione, J. Characterization and Testing of a Novel Sprayable Crosslinked Edible Coating Based on Salmon Gelatin. Coatings 2019, 9, 595. [Google Scholar] [CrossRef] [Green Version]








| Entry | Mw1 | Mw2 (PDI) a,b | MA/HA c (%) | DSUDA d (%) | DS(Bz) e | Yield f (%) |
|---|---|---|---|---|---|---|
| 1 | 15 | 16.4 (1.3) | 30 | 9.4 ± 0.18 | 0.5 ± 0.1 | 94.5 |
| 2 | 15.8 (1.3) | 50 | 16.0 ± 2.1 | 1.6 ± 0.5 | 96.7 | |
| 3 | 13.6 (1.1) | 100 | 32.3 ± 2.1 | 2.5 ± 0.05 | 99.5 | |
| 4 | 16.5 (1.2) | 130 | 35.9 ± 5.0 | 3.6 ± 0.05 | 98.9 | |
| 5 | 110 | 119.8 (1.4) | 30 | 8.1 ± 0.2 | 0.5 ± 0.05 | 95.7 |
| 6 | 117.8 (1.5) | 50 | 16.1 ± 2.0 | 1.7 ± 0.1 | 98.3 | |
| 7 | 125.1 (1.6) | 70 | 20.3 ± 2.5 | 2.2 ± 0.1 | 98.8 | |
| 8 | 125.4 (1.3) | 100 | 32.4 ± 4.8 | 3.0 ± 0.2 | 98.0 | |
| 9 | nd | 130 | 51.8 ± 5.0 | 5.0 ± 0.5 | 95.0 | |
| 10 | 240 | 232.9 (1.6) | 15 | 3.2 ± 0.5 | 0.2 ± 0.1 | 89.6 |
| 11 | 225.4 (1.7) | 20 | 4.7 ± 0.5 | 0.3 ± 0.1 | 96.5 | |
| 12 | 224.2 (1.6) | 30 | 8.2 ± 0.3 | 0.5 ± 0.1 | 94.8 | |
| 13 | 195.5 (1.7) | 50 | 17.2 ± 1.0 | 1.7 ± 0.5 | 91.4 | |
| 14 | 261.8 (1.5) | 90 | 26.5 ± 2.3 | 2.0 ± 0.3 | 92.4 | |
| 15 | 268.4 (1.5) | 100 | 32.8 ± 3.0 | 3.5 ± 0.2 | 92.4 | |
| 16 | nd | 130 | 42.0 ± 2.5 | 3.4 ± 0.2 | 99.6 |
| DSGC (%) | α-Tocopherol | Co(Q10) | Curcumin | |||
|---|---|---|---|---|---|---|
| Drug Loading % (wt./wt.) | EE (%) | Drug Loading % (wt./wt.) | EE (%) | Drug Loading % | EE (%) | |
| 8.2 | 15.12 ± 0.22 | 75.2 ± 0.4 | 6.73 ± 0.25 | 40.8 ± 0.8 | 0.09 ± 0.01 | 25.5 |
| 27.8 | 14.96 ± 0.05 | 74.9 ± 0.6 | 7.46 ± 0.42 | 57.7 ± 0.3 | 0.14 ± 0.01 | 35.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huerta-Ángeles, G.; Brandejsová, M.; Kopecká, K.; Ondreáš, F.; Medek, T.; Židek, O.; Kulhánek, J.; Vagnerová, H.; Velebný, V. Synthesis and Physicochemical Characterization of Undecylenic Acid Grafted to Hyaluronan for Encapsulation of Antioxidants and Chemical Crosslinking. Polymers 2020, 12, 35. https://doi.org/10.3390/polym12010035
Huerta-Ángeles G, Brandejsová M, Kopecká K, Ondreáš F, Medek T, Židek O, Kulhánek J, Vagnerová H, Velebný V. Synthesis and Physicochemical Characterization of Undecylenic Acid Grafted to Hyaluronan for Encapsulation of Antioxidants and Chemical Crosslinking. Polymers. 2020; 12(1):35. https://doi.org/10.3390/polym12010035
Chicago/Turabian StyleHuerta-Ángeles, Gloria, Martina Brandejsová, Kateřina Kopecká, František Ondreáš, Tomáš Medek, Ondrej Židek, Jaromír Kulhánek, Hana Vagnerová, and Vladimir Velebný. 2020. "Synthesis and Physicochemical Characterization of Undecylenic Acid Grafted to Hyaluronan for Encapsulation of Antioxidants and Chemical Crosslinking" Polymers 12, no. 1: 35. https://doi.org/10.3390/polym12010035