Assessment of Photodegradation and Biodegradation of RPU/PIR Foams Modified by Natural Compounds of Plant Origin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Rigid PUR/PIR Foams
2.3. Methods
2.3.1. Analyzing of Foaming Process
2.3.2. Accelerated Aging Test
2.3.3. Aging in a Climate Chamber
2.3.4. Apparent Density
2.3.5. Compressive Strength and Compressive Strength Ratio
2.3.6. Differential Scanning Calorimetry (DSC)
2.3.7. Foam Structure
2.3.8. Chemical Structure
2.3.9. Measurement of Foams Color
2.3.10. Susceptibility on Biodegradation
3. Results and Discussion
3.1. Foaming Process
3.2. Organoleptic Assessment of RPU/PIR Foams
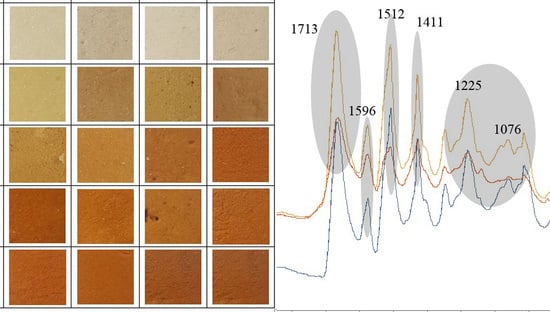
3.3. Color of Foam
3.4. Accelerated Aging Test
3.5. Compressive Strength and Density
3.6. FTIR Analysis
3.7. Differential Scanning Calorimetry
3.8. RPU/PIR Foams Structure
3.9. Biodegradation of RPU/PIR Foams
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eaves, D. Handbook of Polymer Foams; Rapra Technology Ltd.: Shawbury, UK, 2004; p. 289. [Google Scholar]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous organic materials: Strategic design and structure–function correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef]
- Noorani, R. 3D Printing: Technology, Applications, and Selection; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315155494. [Google Scholar]
- Agrawal, A.; Kaura, R.; Walia, R.S. PU foam derived from renewable sources: Perspective on properties enhancement: An overview. Eur. Polym. J. 2017, 95, 255–274. [Google Scholar] [CrossRef]
- Soares, B.; Gama, N.; Freire, C.S.R.; Timmons, A.B.; Silva, B.I.; Neto, C.P.; Ferreira, A. Spent coffee grounds as a renewable source for ecopolyols production. J. Chem. Technol. Biotechnol. 2015, 90, 1480–1488. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Ghasemloua, M.; Daverb, F.; Ivanovac, E.P.; Adhikaria, B. Polyurethanes from seed oil-based polyols: A review of synthesis, mechanical and thermal properties. Ind. Crop. Prod. 2019, 142, 111841–111858. [Google Scholar] [CrossRef]
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and disposal methods for polyurethane foam wastes. The 7th International Conference on Waste Management and Technology. Procedia Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Tomaszewska, E.; Liszkowska, J. New bio-polyol based on white mustard seed oil for rigid PUR-PIR foams. Pol. J. Chem. Technol. 2018, 20, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.; Gama, N.V.; Soares, B.; Freire, C.S.R.; Barros-Timmons, A.; Brandão, I.; Silva, R.; Neto, C.P. Method for Production of Rigid Polyurethane Foams Using Unrefined Crude Glycerol. Patent Application 107,711, 12 June 2014. [Google Scholar]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Tomaszewska, E.; Liszkowska, J. Oenothera biennis seed oil as an alternative raw material for production of bio-polyol for rigid polyurethane-polyisocyanurate foams. Ind. Crop. Prod. 2018, 126, 208–217. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Isbrandt, M. Effect of Evening Primrose Oil-Based Polyol on the Properties of Rigid Polyurethane–Polyisocyanurate Foams for Thermal Insulation. Polymers 2018, 10, 1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prociak, A.; Pielichowski, J. Characteristics of rigid polyurethane boilings in the aspect of new porophors application. Czas. Tech. 1998, R95, 26–52. [Google Scholar]
- Żuchowska, D. Construction Polymers; WNT: Warsaw, Poland, 2000. [Google Scholar]
- Aranguren, M.I.; González, J.F.; Mosiewicki, M.A. Biodegradation of a vegetable oil based polyurethane and wood flour composites. Polym. Test. 2012, 31, 7–15. [Google Scholar] [CrossRef]
- Borowicz, M. New bio-polyol based on white mustard seed (Sinapis alba) as an alternative raw material for the polyurethane industry. Polimery 2018, 63, 38–43. [Google Scholar] [CrossRef]
- Mucha, M. Polimery a Ekologia (Eng. Polymers and Ecology); WU Politechnika Łódzka: Łódź, Poland, 2002. [Google Scholar]
- Lubczak, R.; Sczęch, D. Polyurethane foams with starch. J. Chem. Techol. Biotechnol. 2019, 94, 109–119. [Google Scholar] [CrossRef]
- Gama, N.V.; Silva, R.; Costa, M.; Barros-Timmons, A.; Ferreira, A. Statistical evaluation of the effect of formulation on the properties of crude glycerol polyurethane foams. Polym. Test. 2016, 56, 200–206. [Google Scholar] [CrossRef]
- Sousa, A.F.; Matos, M.; Pinto, R.J.B.; Freire, C.S.R.; Silvestre, A.J.D. One-pot synthesis of biofoams from castor oil and cellulose microfibers for energy absorption impact materials. Cellulose 2014, 21, 1723–1733. [Google Scholar] [CrossRef]
- Veronese, V.B.; Menger, R.K.; Forte, M.M.C.; Petzhold, C.L. Rigid polyurethane foam based on modified vegetable oil. J. Appl. Polym. Sci. 2011, 120, 530–537. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryński, B.; Borowicz, M.; Liszkowska, J. New polyurethane materials containing biofiller. Polimery 2015, 60, 586–591. [Google Scholar] [CrossRef]
- Pavier, C.; Gandini, A. Urethanes and polyurethanes from oxypropylated sugar beet pulp I. Kinetic study in solution. Eur. Polym. J. 2000, 36, 1653–1658. [Google Scholar] [CrossRef]
- Gandini, A.; Neto, C.P.; Silvestre, A.J.D. Suberin: A promising renewable resource for novel macromolecular materials. Prog. Polym. Sci. 2006, 31, 878–892. [Google Scholar] [CrossRef]
- Soares, B.; Gama, N.; Freire, C.; Barros-Timmons, A.; Brandão, I.; Silva, R.; Pascoal, N.C.; Ferreira, A. Ecopolyols production from industrial cork powder via acid liquefaction using polyhydric alcohols. ACS Sustain. Chem. Eng. 2014, 2, 846–854. [Google Scholar] [CrossRef]
- Fernandes, S.; Freire, C.S.R.; Neto, C.P.; Gandini, A. The bulk oxypropylation of chitin and chitosan and the characterization of the ensuing polyols. Green Chem. 2008, 10, 93–97. [Google Scholar] [CrossRef]
- De Menezes, A.J.; Pasquini, D.; Curvelo, A.A.S.; Gandini, A. Novel thermoplastic materials based on the outer-shell oxypropylation of corn starch granules. Biomacromolecules 2007, 8, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoshioka, M.; Shiraishi, N. Liquefaction of corn bran (CB) in the presence of alcohols and preparation of polyurethane foam from its liquefied polyol. J. Appl. Polym. Sci. 2000, 78, 319–325. [Google Scholar] [CrossRef]
- Liang, L.; Mao, Z.; Li, Y.; Wan, C.; Wang, T.; Lianhui, Z.; Lingyan, Z. Liquefaction of crop residues for polyol production. Bioresources 2006, 1, 248–256. [Google Scholar]
- Yan, Y.; Pang, H.; Yang, X.; Zhang, R.; Liao, B. Preparation and characterization of water-blown polyurethane foam from liquefied cornstalk polyol. J. Appl. Polym. Sci. 2008, 110, 1099–1111. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, F.; Luo, C.; Xiong, L.; Chen, X. Liquefaction and characterization of acid hydrolysis residue of corncob in polyhydric alcohols. Ind. Crop. Prod. 2012, 39, 47–51. [Google Scholar] [CrossRef]
- Pan, X.; Saddler, J.N. Effect of replacing polyol by organosolv and kraft lignin on the property and structure of rigid polyurethane foam. Biotechnol. Biofuels 2013, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Ruan, X.; Cheng, X.; Lü, Q. Liquefaction of lignin by polyethyleneglycol and glycerol. Bioresour. Technol. 2011, 102, 3581–3583. [Google Scholar] [CrossRef]
- D’Souza, J.; George, B.; Camargo, R.; Yan, N. Synthesis and characterization of bio-polyols through the oxypropylation of bark and alkaline extracts of bark. Ind. Crop. Prod. 2015, 76, 1–11. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryński, B.; Borowicz, M.; Liszkowska, J. Composites of rigid polyurethane-polyisocyanurate foams with oak bark. Polimery 2017, 62, 666–672. [Google Scholar] [CrossRef]
- Zheng, Z.; Pan, H.; Huang, Y.; Chung, Y.; Zhang, X.; Feng, H. Rapid liquefaction of wood in polyhydric alcohols under microwave heating and its liquefied products for preparation of rigid polyurethane foam. Open Mater. Sci. J. 2011, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Zheng, Z.; Hse, C. Microwave-assisted liquefaction of wood with polyhydric alcohols and its application in preparation of polyurethane (PU) foams. Eur. J. Wood. Wood. Prod. 2012, 70, 461–470. [Google Scholar] [CrossRef]
- Chen, F.; Lu, Z. Liquefaction of wheat straw and preparation of rigid polyurethane foam from the liquefaction products. J. Appl. Polym. Sci. 2009, 111, 508–516. [Google Scholar] [CrossRef]
- Hassan, M.; Shukry, N. Polyhydric alcohol liquefaction of some lignocellulosic agricultural residues. Ind. Crop. Prod. 2008, 27, 33–38. [Google Scholar] [CrossRef]
- Briones, R.; Serrano, L.; Labidi, J. Valorization of some lignocellulosic agro-industrial residues to obtain biopolyols. J. Chem. Technol. Biotechnol. 2012, 87, 244–249. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Szulc, J.; Kloziński, A. Poly(lactic acid) green composites filled with linseed cake as an agricultural waste filler. Influence of oil content within the filler on the rheological behavior. J. Appl. Polym. Sci. 2019, 136, 47650–47672. [Google Scholar] [CrossRef]
- Nejman, M.B. (Ed.) Collective Work: Aging and Polymer Stabilization (Starzenie i Stabilizacja Polimerów); WNT: Warsaw, Poland, 1996. [Google Scholar]
- White, J.R. Polymer ageing: Physics, chemistry or engineering? Time to reflect. C. R. Chim. 2006, 9, 1396–1408. [Google Scholar] [CrossRef]
- Liszkowska, J.; Moraczewski, K.; Borowicz, M.; Paciorek-Sadowska, J.; Czupryński, B.; Isbrandt, M. The Eect of Accelerated Aging Conditions on the Properties of Rigid Polyurethane-Polyisocyanurate Foams Modified by Cinnamon Extract. Appl. Sci. 2019, 9, 2663. [Google Scholar] [CrossRef] [Green Version]
- Czupryński, B. Issues in Chemistry and Technology of Polyurethanes; WAB: Bydgoszcz, Poland, 2004. [Google Scholar]
- Sobków, M.; Czaja, K. Influence of accelered ageing conditons on the process of polyolefines degradation. Polimery 2003, 48, 627–632. [Google Scholar] [CrossRef]
- Rajlakshmi, N.; Tarkes, D.P.; Alok, S. A computational and experimental investigation on thermal conductivity of particle reinforced epoxy composites. Comput. Mater. Sci. 2010, 48, 576–581. [Google Scholar]
- Bal, K.; Mielniczuk, Z. Methods for testing the migration of harmful substances from packaging to food (pol. Metody badania migracji szkodliwych substancji z opakowań do żywności). Pap. Rev. 2010, 66, 459–462. [Google Scholar]
- Latos, M.; Masek, A.; Zaborski, M. Migration of auxiliary modifying agents from polymeric packaging materials (in polish: Migracja pomocniczych środków modyfikujących z polimerowych materiałow opakowaniowych). Plast. Process. 2017, 4, 364–369. [Google Scholar]
- Commission Regulation (EU) No. 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Available online: http://data.europa.eu/eli/reg/2011/10/oj (accessed on 23 December 2019).
- Technical Card of Cinnamon, Agrema Sp. z o.o.
- Danielewska, A.; Maciąg, K. (Eds.) Selected Plant Properties—The Latest Reports; WN TYGIEL sp. z o. o.: Lublin, Poland, 2019. [Google Scholar]
- Yi-Fang, C.H. (Ed.) Coffee: Emerging Health Effects and Disease Prevention; Jonh Wiley and Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Czaplińska, M.; Czepas, J.; Gwoździński, K. Structure, antioxidant and anti-cancer properties flavonoid bike. Adv. Biochem. 2012, 58, 235–244. [Google Scholar]
- Wirpsza, Z. Polyurethanes: Chemistry, Technology, Application; WNT: Warsaw, Poland, 1991. (In Polish) [Google Scholar]
- Liszkowska, J.; Czupryński, B.; Paciorek-Sadowska, J. Thermal properties of polyurethane-polyisocyanurate (PUR-PIR) foams modified with tris(5-hydroxypentyl)-2-hydroxypropane-1,2,3-tricarboxylate. J. Adv. Chem. Eng. 2016, 6, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Czupryński, B.; Liszkowska, J.; Paciorek-Sadowska, J. Modification of rigid polyurethane-polyisocyanurate foam selected powder fillers. Polimery 2008, 3, 48–52. [Google Scholar]
- ASTM International (American Society for Testing and Materials). Standard Practice for Polyurethane Raw Materials: Polyurethane Foam Cup Test; ASTM Standard D7487–13e1; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Broniewski, T.; Kapko, J.; Płaczek, W.; Thomalla, J. Test Methods and Evaluation of Plastic Properties; WNT: Warsaw, Poland, 2000. [Google Scholar]
- Es-tor. Operating Instructions for the Climate Chamber Model CCK-40/300 NG.
- Bajda, M.; Pogoda, M. Study of the effect of conveyor belt accelerated heat aging on its strength. Transport 2016, 1, 16–22. [Google Scholar]
- Szczepaniak, W. Instrumental Methods in Chemical Analysis; PWN: Warsaw, Poland, 2011. [Google Scholar]
- Borowicz, M. Synthesis and Application of New Bio-Polyols Based on Vegetable Raw Materials for the Production of Bio-Composites in the Form of Rigid Polyurethane-Polyisocyanurate Foams. Ph.D. Thesis, West Pomeranian University of Technology, Szczecin, Poland, 2019. (In Polish). [Google Scholar]
- Prociak, A. High-Tech Insulation Polyurethane Materials; WU PK: Cracow, Poland, 2008. [Google Scholar]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Liszkowska, J.; Tomaszewska, E. Application of halloysite as filler in the production of rigid PUR-PIR foams. Polimery 2018, 63, 185–190. [Google Scholar] [CrossRef]
- Singh, S.N. Blowing Agents for Polyurethane Foams; Rapra Technology: Shawbury, UK, 2002; Volume 12, ISBN 1859573215. [Google Scholar]
- Levchik, S.V.; Weil, E.D. Thermal decomposition, combustion and fire-retardancy of polyurethanes-a review of the recent literature. Polym. Int. 2004, 53, 1585–1610. [Google Scholar] [CrossRef]
- Rojek, M. Methodology of diagnostic tests of layered composite materials with a polymer matrix. Open Access Libr. 2011, 2, 11–31. [Google Scholar]
- Pączkowski, J. Photochemistry of Polymers. In Theory and Applications; WU UMK: Toruń, Poland, 2003. [Google Scholar]
- Gama, N.V.; Silva, R.; Mohseni, F.; Davarpanah, A.; Amaral, V.S.; Ferreira, A.; Barros-Tommons, A. Enhancement of physical and reaction to fire properties of crude glycerol polyurethane foams filled with expanded graphite. Polym. Test. 2018, 69, 199–207. [Google Scholar] [CrossRef]
- Liszkowska, J. The effect of ground coffee on the mechanical and application properties of rigid polyurethane-polyisocyanurate foams. Polimery 2018, 63, 305–310. [Google Scholar] [CrossRef]
- Szumera, M. Characteristics of selected thermal methods. Part 1. LAB Lab. Appar. Res. 2012, 17, 28–34. [Google Scholar]
- Liszkowska, J.; Paciorek-Sadowska, J.; Czupryński, B. Temperature stability and thermal properties of polyurethane-polyisocyanurate foams obtained using products of citric acid condensation. Polimery 2018, 63, 503–514. [Google Scholar] [CrossRef]
- Czupryński, B.; Paciorek-Sadowska, J.; Liszkowska, J. Heat and thermal reistance of rigid polyurethane-polyisocyanurate foams with addition of polyethylene, polypropylene and foamed polystyrene recyclates. Inż. I Apar. Chem. 2008, 4, 15–16. [Google Scholar]
- Paciorek-Sadowska, J. Research on the Influence of Boric Acid and N, N- (Dihydroxymethyl) Urea Derivatives on the Properties of Rigid Polyurethane-Polyisocyanurate Foams; WU UKW: Bydgoszcz, Poland, 2011. [Google Scholar]
- Liszkowska, J. Properties of Rigid PUR-PIR Foams Obtained with the Condensation of Citric Acid with Diols and Selected Glycolysates; WU UKW: Bydgoszcz, Poland, 2016. [Google Scholar]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. Effect of foam density on the properties of water blown rigid polyurethane foam. J. Appl. Polym. Sci. 2008, 8, 1810–1817. [Google Scholar] [CrossRef]
- Tosin, M.; Pischedda, A.; Degli-Innocenti, F. Biodegradation kinetics in soil of a multi-constituent biodegradable Plastic. Polym. Degrad. Stab. 2019, 166, 213–218. [Google Scholar] [CrossRef]
- Grabowska, B. Biodegradation of polymer materials. Arch. Foundry Eng. 2010, 10, 57–60. [Google Scholar]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnol. Res. Int. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Zheng Zhang, Z.; Ortiz, O.; Goyal, R.; Kohn, J. Handbook of Polymer Applications in Medicine and Medical Devices; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar]










| Compound | Content in C10 Foam | Content in KZ10 Foam | Content in EK10 Foam |
|---|---|---|---|
| Polyfenols (e.g., chlorogenic acid, flavonoids, phenol acids) | 5.0% | 45.0% | 5.0% |
| Minerals | <2.0 ppm | 4.4% | <0.3 ppm |
| Carrier to active substances (e.g., cellulose, lignin, polysaccharides) | about 94.9% | about 50.6% | about 94.9% |
| Foam | Filler (wt %) | ||
|---|---|---|---|
| Cinnamon | Green Coffee | Cocoa | |
| W_0 | 0 | 0 | 0 |
| C10_0 | 10 | 0 | 0 |
| KZ10_0 | 0 | 10 | 0 |
| EK10_0 | 0 | 0 | 10 |
| Foam | Cream Time (s) | Free Rise Time (s) | String Gel Time (s) | Tack Free Time (s) | Tmax (°C) |
|---|---|---|---|---|---|
| W_0 | 8 | 34 | 23 | 21 | 126 |
| C10_0 | 10 | 48 | 30 | 25 | 146 |
| KZ10_0 | 10 | 58 | 29 | 23 | 165 |
| EK10_0 | 10 | 45 | 31 | 24 | 152 |
| Foam | ∆l (%) | ∆V (%) | ∆m (%) |
|---|---|---|---|
| W_2 | +0.39 ± 0.01 | −1.77 ± 0.01 | +3.27 ± 0.01 |
| C10_2 | +0.80 ± 0.01 | −2.94 ± 0.01 | +4.35 ± 0.01 |
| KZ10_2 | −0.12 ± 0.01 | +0.08 ± 0.01 | −0.56 ± 0.01 |
| EK10_2 | +0.20 ± 0.01 | −2.39 ± 0.01 | +0.67 ± 0.01 |
| Foam | d (kg/m3) | W0 (kPa) | W7 (kPa) | W14 (kPa) | W21 (kPa) | CV1 (%) | CV2 (%) | CV3 (%) |
|---|---|---|---|---|---|---|---|---|
| W_0 | 39.7 ± 0.1 | 251.6 ± 1.4 | 151.8 ± 1.3 | 143.8 ± 1.3 | 140.6 ± 1.3 | 60.3 ± 0.5 | 57.2 ± 0.4 | 55.9 ± 0.4 |
| C10_0 | 35.7 ± 0.1 | 160.4 ± 1.3 | 143.9 ± 1.3 | 132.5 ± 1.3 | 68.1 ± 1.1 | 89.7 ± 0.5 | 82.6 ± 0.4 | 42.5 ± 0.3 |
| KZ10_0 | 42.9 ± 0.1 | 227.3 ± 1.4 | 181.9 ± 1.4 | 131.1 ± 1.2 | 120.9 ± 1.2 | 80.0 ± 0.5 | 57.7 ± 0.4 | 53.2 ± 0.4 |
| EK10_0 | 35.7 ± 0.1 | 178.3 ± 1.4 | 153.9 ± 1.3 | 113.5 ± 1.3 | 95.1 ± 1.1 | 86.3 ± 0.5 | 63.7 ± 0.4 | 53.3 ± 0.4 |
| Band (cm−1) | Bond |
|---|---|
| 3325 | N–H |
| 2930 | C–H |
| 2276 | –N=C=O |
| 2137 | –N=C=N– |
| 1713 | –C=O in urethane bond |
| 1596 | N–H |
| 1512 | N–H |
| 1411 | Isocyanurate ring |
| 1225 | C=N in trimer |
| 1076 | C–O |
| Foam | Peak P1 | Peak P2 | Peak P3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tonset, (°C) | Tmax1 (°C) | Tk1 (°C) | H1 (J/g) | Tonset2 (°C) | Tmax2 (°C) | Tk2 (°C) | H2 (J/g) | Tonset3 (°C) | Tmax3 (°C) | Tk3 (°C) | H3 (J/g) | |
| W_0 | 21.7 | 71.8 | 146.6 | 25.75 | 278.6 | 294.8 | 325.3 | 44.86 | 326.3 | 330.4 | 347.3 | 5.08 |
| W_7 | 54.7 | 91.3 | 179.8 | 41.76 | 271.5 | 289.0 | 314.4 | 18.95 | 318.2 | 323.6 | 342.2 | 4.73 |
| W_21 | 14.6 | 64.6 | 106.2 | 68.10 | 166.1 | 253.2 | 294.6 | 87.41 | 322.4 | 347.8 | 393.3 | 16.68 |
| C10_0 | 46.7 | 86.4 | 139.4 | 33.38 | 261.9 | 274.5 | 319.1 | 35.60 | 324.0 | 329.8 | 352.7 | 6.06 |
| C10_7 | 39.7 | 93.9 | 133.9 | 47.54 | 259.7 | 285.3 | 313.0 | 13.83 | 331.2 | 337.5 | 358.8 | 5.24 |
| C10_21 | 10.2 | 63.2 | 146.6 | 98.32 | 263.5 | 286.5 | 323.8 | 14.32 | 332.6 | 353.9 | 396.1 | 12.05 |
| KZ10_0 | 15.9 | 68.0 | 96.0 | 19.56 | 213.0 | 247.7 | 297.8 | 18.40 | 304.3 | 331.9 | 352.0 | 17.17 |
| KZ10_7 | 21.3 | 84.8 | 139.4 | 44.23 | 200.7 | 263.2 | 291.0 | 22.12 | 295.4 | 319.1 | 337.2 | 6.02 |
| KZ10_21 | 17.3 | 66.7 | 109.8 | 81.72 | 231.1 | 240.1 | 292.2 | 24.02 | 328.9 | 351.1 | 385.9 | 10.81 |
| EK10_0 | 50.3 | 96.6 | 150.6 | 47.43 | 260.6 | 274.6 | 299.3 | 36.89 | 304.3 | 311.6 | 363.7 | 17.50 |
| EK10_14 | 23.4 | 70.3 | 114.6 | 79.78 | 163.6 | 249.4 | 291.8 | 63.97 | 309.7 | 348.5 | 394.8 | 19.86 |
| EK10_21 | 51.9 | 107.5 | 153.3 | 70.11 | 262.3 | 272.8 | 289.1 | 1.97 | 293.5 | 304.5 | 317.4 | 5.81 |
| Foam Symbol | Cell/Hole Height (µm) | Cell/Hole Width (µm) | Anisotrophy Coefficient (-) | Cell/Hole Surface Area (mm2) | Content of Cell/Hole Per Area Unit (cell/mm2) |
|---|---|---|---|---|---|
| W_0 | 606.6 ± 2.2 | 540.7 ± 0.2 | 1.12 ± 0.01 | 0.258 ± 0.001 | 3.88 ± 0.01 |
| W_7 | 367.7 ± 1.1 | 340.9 ± 0.1 | 1.08 ± 0.01 | 0.132 ± 0.001 | 7.58 ± 0.01 |
| W21 | 267.8 ± 1.1 | 219.6 ± 0.1 | 1.22 ± 0.01 | 0.046 ± 0.001 | 21.74 ± 0.01 |
| C10_0 | 346.7 ± 1.1 | 324.9 ± 0.1 | 1.11 ± 0.01 | 0.177 ± 0.001 | 5.65 ± 0.01 |
| C10_7 | 311.4 ± 1.1 | 299.0 ± 0.1 | 1.04 ± 0.01 | 0.146 ± 0.001 | 6.85 ± 0.01 |
| C10_21 | 218.0 ± 1.1 | 226.8 ± 0.1 | 0.96 ± 0.01 | 0.038 ± 0.001 | 26.32 ± 0.01 |
| KZ10_0 | 303.4 ± 1.1 | 279.2 ± 0.1 | 1.09 ± 0.01 | 0.133 ± 0.001 | 7.52 ± 0.01 |
| KZ10_7 | 289.1 ± 1.1 | 277.5 ± 0.1 | 1.04 ± 0.01 | 0.126 ± 0.001 | 7.94 ± 0.01 |
| KZ10_21 | 220.7 ± 1.1 | 222.3 ± 0.1 | 0.99 ± 0.01 | 0.384 ± 0.001 | 26.0 ± 0.01 |
| EK10_0 | 356.4 ± 1.1 | 351.2 ± 0.1 | 1.01 ± 0.01 | 0.197 ± 0.001 | 5.08 ± 0.01 |
| EK10_7 | 346.7 ± 1.1 | 344.9 ± 0.1 | 1.00 ± 0.01 | 0.188 ± 0.001 | 5.32 ± 0.01 |
| EK_21 | 208.7 ± 1.1 | 193.3 ± 0.1 | 1.08 ± 0.01 | 0.032 ± 0.001 | 31.25 ± 0.01 |
| Foam | C | H | O | Si | N | P | Cl | K |
|---|---|---|---|---|---|---|---|---|
| W_0 | 0.627 ± 0.001 | 0.058 ± 0.001 | 0.183 ± 0.001 | 0.005 ± 0.001 | 0.067 ± 0.001 | 0.013 ± 0.001 | 0.045 ± 0.001 | 0.003 ± 0.001 |
| C10_0 | 0.610 ± 0.001 | 0.058 ± 0.001 | 0.209 ± 0.001 | 0.005 ± 0.001 | 0.062 ± 0.001 | 0.012 ± 0.001 | 0.041 ± 0.001 | 0.002 ± 0.001 |
| KZ10_0 | 0.610 ± 0.001 | 0.058 ± 0.001 | 0.209 ± 0.001 | 0.005 ± 0.001 | 0.062 ± 0.001 | 0.012 ± 0.001 | 0.041 ± 0.001 | 0.002 ± 0.001 |
| EK10_0 | 0.622 ± 0.001 | 0.057 ± 0.001 | 0.199 ± 0.001 | 0.005 ± 0.001 | 0.062 ± 0.001 | 0.012 ± 0.001 | 0.041 ± 0.001 | 0.002 ± 0.001 |
| Foam | Sample Weight (g) | BOD28 (mg/L) | TOD (mg/L) | Dt (%) |
|---|---|---|---|---|
| W_0 | 0.203 ± 0.001 | 7.0 ± 0.1 | 81.27 ± 0.01 | 8.30 ± 0.01 |
| C10_0 | 0.211 ± 0.001 | 57.7 ± 0.1 | 73.36 ± 0.01 | 78.65 ± 0.01 |
| KZ10_0 | 0.207 ± 0.001 | 53.5 ± 0.1 | 76.26 ± 0.01 | 70.16 ± 0.01 |
| EK10_0 | 0.223 ± 0.001 | 42.2 ± 0.1 | 71.77 ± 0.01 | 58.80 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liszkowska, J.; Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M.; Czupryński, B.; Moraczewski, K. Assessment of Photodegradation and Biodegradation of RPU/PIR Foams Modified by Natural Compounds of Plant Origin. Polymers 2020, 12, 33. https://doi.org/10.3390/polym12010033
Liszkowska J, Borowicz M, Paciorek-Sadowska J, Isbrandt M, Czupryński B, Moraczewski K. Assessment of Photodegradation and Biodegradation of RPU/PIR Foams Modified by Natural Compounds of Plant Origin. Polymers. 2020; 12(1):33. https://doi.org/10.3390/polym12010033
Chicago/Turabian StyleLiszkowska, Joanna, Marcin Borowicz, Joanna Paciorek-Sadowska, Marek Isbrandt, Bogusław Czupryński, and Krzysztof Moraczewski. 2020. "Assessment of Photodegradation and Biodegradation of RPU/PIR Foams Modified by Natural Compounds of Plant Origin" Polymers 12, no. 1: 33. https://doi.org/10.3390/polym12010033