Properties of Luffa Fiber Reinforced PHBV Biodegradable Composites
Abstract
:1. Introduction
2. Materials
2.1. Chemical Treatment of the Fibers
2.2. Fabrication of Composites
2.3. Characterization of Fibers and Composites
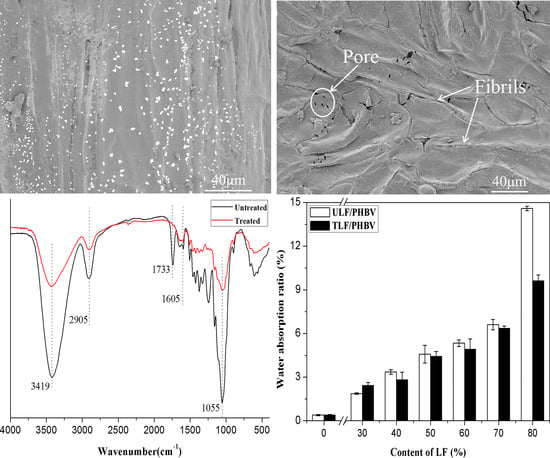
3. Results and Discussion
3.1. Properties of LF before and after Treatment
3.2. Properties of Composite Materials
3.2.1. FTIR Spectra of Composites
3.2.2. Water Absorptivity
3.2.3. Thermogravimetry
3.2.4. Mechanical Property
3.2.5. Mechanical Properties after Soaking
3.2.6. SEM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saeed, A.; Iqbal, M. Loofa (Luffa cylindrica) sponge: Review of development of the biomatrix as a tool for biotechnological applications. Biotechnol. Prog. 2013, 29, 573–600. [Google Scholar] [CrossRef] [PubMed]
- Oboh, I.O.; Aluyor, E.O. Luffa cylindrica-an emerging cash crop. Afr. J. Agric. Res. 2009, 4, 684–688. [Google Scholar] [CrossRef]
- Mazali, I.O.; Alves, O.L. Morphosynthesis: High fidelity inorganic replica of the fibrous network of loofa sponge (Luffa cylindrica). An. Acad. Bras. Ciências 2005, 77, 25–31. [Google Scholar] [CrossRef]
- Chen, J.P.; Lin, T.C. Loofa sponge as a scaffold for culture of rat hepatocytes. Biotechnol. Prog. 2010, 21, 315–319. [Google Scholar] [CrossRef]
- Watanabe, K.; Minami, Y.; Funatsu, G. Isolation and partial characterization of three protein synthesis inhibitory proteins from the seeds of luffa of three protein synthesis inhibitory proteins from the seeds of Luffa cylindrical. Agric. Biol. Chem. 1990, 54, 2085–2092. [Google Scholar] [CrossRef]
- Roble, N.; Ogbonna, J.; Tanaka, H. A novel circulating loop bioreactor with cells immobilized in loofa (Luffa cylindrica) sponge for the bioconversion of raw cassava starch to ethanol. Appl. Microbiol. Biotechnol. 2003, 60, 671–678. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, F.; Guo, Y.; Hu, D.; Zhu, Z.; Zhang, K.; Zhu, S. A novel mattress filling material comprising of luffa fibers and EVA resin. Ind. Crop. Prod. 2018, 124, 213–215. [Google Scholar] [CrossRef]
- Parida, C.; Patra, S.; Mohanta, K.L.; Dash, S.K.; Parashar, S.K.S. Study of Dielectric Properties of Biodegradable Composites Using (Poly)Lactic Acid and Luffa Fiber; American Institute of Physics Conference Series; AIP Publishing: Melville, NY, USA, 2017; p. 1832. [Google Scholar] [CrossRef]
- Kakar, A.; Jayamani, E.; Bakri, M.K.b.; Heng, S.K. Heat Treated Luffa-PLA Composites: Effect of Cyclic Moisture Absorption and Desorption on the Mechanical Properties. Mater. Sci. Forum 2018, 917, 42–46. [Google Scholar] [CrossRef]
- Papanicolaou, G.C.; Psarra, E.; Anastasiou, D. Manufacturing and mechanical response optimization of epoxy resin/Luffa Cylindrica composite. J. Appl. Polym. Sci. 2015, 132, 22. [Google Scholar] [CrossRef]
- Essabir, H.; Hilali, E.; Minor, H.E.; Bensalah, M.O.; Bouhfid, R.; Qaiss, A. Mechanical and Thermal Properties of Polymer Composite Based on Natural Fibers: Moroccan Luffa Sponge/High Density Polyethylene. J. Financ. Regul. Compliance 2015, 9, 350–357. [Google Scholar] [CrossRef]
- Lai, S.M.; Kao, Y.H.; Liu, Y.K.; Chiu, F.C. Preparation and properties of luffa fiber- and kenaf fiber-filled poly (butylene succinate-co-lactate)/starch blend-based biocomposites. Polym. Test. 2016, 50, 191–199. [Google Scholar] [CrossRef]
- Escocio, V.A.; Visconte, L.L.Y.; Cavalcante, A.D.P.; Furtado, A.M.S.; Pacheco, E.B.A.V. Study of mechanical and morphological properties of bio-based polyethylene (HDPE) and sponge-gourds (Luffa-cylindrica) agroresidue composites. AIP Conf. 2015, 1664, 060012. [Google Scholar] [CrossRef]
- Xu, S. Effect of Alkaline Treatment on the Interfacial Properties of Biodegradable Composites made from Henequen fibres and Poly(Hydroxybutyrate-Co-Valerate) Resin. Tech. Text. 2002, 12, 27–30. [Google Scholar]
- Eke, G.; Kuzmina, A.M.; Goreva, A.V.; Shishatskaya, E.I.; Hasirci, N.; Hasirci, V. In vitro and transdermal penetration of PHBV micro/nanoparticles. J. Mater. Sci. Mater. Med. 2014, 25, 1471–1481. [Google Scholar] [CrossRef]
- Vilos, C.; Constandil, L.; Rodas, P.I.; Cantin, M.; Zepeda, K.; Herrera, N.; Velasquez, L.A. Evaluation of ceftiofur–PHBV microparticles in rats. Drug Des. Dev. Ther. 2014, 8, 651–666. [Google Scholar] [CrossRef]
- Hassaini, L.; Kaci, M.; Benhamida, A.; Bruzaud, S.; Pillin, I.; Grohens, Y. The effects of PHBV-g-MA compatibilizer on morphology and properties of poly(3-hydroxybutyrate-Co-3-hydroxyvalerate)/olive husk flour composites. J. Adhes. Sci. Technol. 2016, 30, 2061–2080. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lui, W.B.; Peng, J. Optimization of Extrusion Variables and Maleic Anhydride Content on Biopolymer Blends Based on Poly(hydroxybutyrate-co-hydroxyvalerate)/Poly (vinyl acetate) with Tapioca Starch. Polymers 2018, 10, 827. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Bhat, A.H.; Ahmad, F. Polymer blend of PLA/PHBV based bionanocomposites reinforced with nanocrystalline cellulose for potential application as packaging material. Carbohydr. Polym. 2017, 157, 1323–1332. [Google Scholar] [CrossRef]
- Peterson, S.; Jayaraman, K.; Bhattacharyya, D. Forming performance and biodegradability of woodfiber-BiopolTM composites. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1123–1134. [Google Scholar] [CrossRef]
- Luo, S.; Netravali, A.N. Mechanical and thermal properties of environment-friendly “Green” composites made from pineapple leaf fibers and poly (hydroxybuty rate-cohydroxyvalerate). Compos. Part A Appl. Sci. Manuf. 1999, 20, 367–378. [Google Scholar] [CrossRef]
- Luo, S.; Netravali, A.N. Interfacial and mechanical properties of environment-friendly “Green” composites made from pineapple leaf fibers and poly (hydroxybuty rate-cohydroxyvalerate) resin. J. Mater. Sci. 1999, 34, 3709–3719. [Google Scholar] [CrossRef]
- Singh, S.; Mohanty, A.K.; Sugie, T.; Takai, Y.; Hamada, H. Renewable resource based biocomposites from natural fiber and polyhydroxybutyrate-co-valerate (PHBV) bioplastic. Compos. Part A Appl. Sci. Manuf. 2008, 39, 875–886. [Google Scholar] [CrossRef]
- Masmoudi, F.; Bessadok, A.; Dammak, M.; Jaziri, M.; Ammar, E. Biodegradable packaging materials conception based on starch and polylactic acid (PLA) reinforced with cellulose. Environ. Sci. Pollut. Res. 2016, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.F.; Gafur, M.A.; Ahmed, A.N.; Dhar, S.A. Effect of Chemical Modifications on Surface Morphological, Structural, Mechanical, and Thermal Properties of Sponge-gourd Natural Fiber. Fibers Polym. 2018, 19, 31–40. [Google Scholar] [CrossRef]
- Aggarwal, P.K.; Raghu, N.; Karmarkar, A.; Chuahan, S. Jute-polypropylene composites using m-TMI-grafted polypropylene as a coupling agent. Mater. Des. 2013, 43, 112–117. [Google Scholar] [CrossRef]
- Wei, W.; Yu, Z.; Zhu, M.; Chen, Y. Effect of graft modification with poly(N-vinylpyrrolidone) on thermal and mechanical properties of poly (3-hydroxybutyrate-co-3-hydroxyvalerate). J. Appl. Polym. Sci. 2010, 109, 1699–1707. [Google Scholar] [CrossRef]
- Chen, Y.; Su, N.; Zhang, K.; Zhu, S.; Zhao, L.; Fang, F.; Ren, L.; Guo, Y. In-Depth Analysis of the Structure and Properties of Two Varieties of Natural Luffa Sponge Fibers. Materials 2017, 10, 479. [Google Scholar] [CrossRef]
- Chen, Y.; Su, N.; Zhang, K.; Zhu, S.; Zhu, Z.; Qin, W.; Yang, Y.; Shi, Y.; Fan, S.; Wang, Z.; et al. Effect of fiber surface treatment on structure, moisture absorption and mechanical properties of luffa sponge fiber bundles. Ind. Crop. Prod. 2018, 123, 341–352. [Google Scholar] [CrossRef]
- Boynard, C.A.; D’Almeida, J.R.M. Water absorption by sponge gourd (luffa cylindrica)-polyester composite materials. J. Mater. Sci. Lett. 1999, 18, 1789–1791. [Google Scholar] [CrossRef]
- Yu, M.; He, C.; Zhang, H.; Hou, R.; Xue, J. Effect of different pretreatments on tribological properties of wheat straw/polypropylene composites. Trans. Chin. Soc. Agric. Mach. 2013, 44, 138–143. [Google Scholar]
- Wu, Y.; Li, X.; Qin, Z.; Qing, Y.; Luo, S. Study on fabricatiopn and properties of wood fiber/poly-lactic acid biodegradable composites. J. Cent. South Univ. For. Technol. 2012, 23. [Google Scholar]
- Paiva, M.C.; Ammar, I.; Campos, A.R.; Cheikh, R.B.; Cunha, A.M. Alfa fibres: Mechanical, morphological and interfacial characterization. Compos. Sci. Technol. 2007, 67, 1132–1138. [Google Scholar] [CrossRef]
- Kiruthika, A.V.; Veluraja, K. Experimental studies on the physico-chemical properties of banana fibre from various varieties. Fibers Polym. 2009, 10, 193–199. [Google Scholar] [CrossRef]
- Biagiotti, J.; Puglia, D.; Torre, L.; Kenny, J.M.; Arbelaiz, A.; Cantero, G.; Marieta, C.; Llano-Ponte, R.; Mondragon, I. A systematic investigation on the influence of the chemical treatment of natural fibers on the properties of their polymer matrix composites. Polym. Compos. 2010, 25, 470–479. [Google Scholar] [CrossRef]
- Saravanakumar, S.S.; Kumaravel, A.; Nagarajan, T.; Sudhakar, P.; Baskaran, R. Characterization of a novel natural cellulosic fiber from Prosopis juliflora bark. Carbohydr. Polym. 2013, 92, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, L.; Sampath, P.S.; Mylsamy, K. Investigation of physical, chemical and mechanical properties of raw and alkali treated Borassus fruit fiber. Compos. Part B 2012, 43, 3044–3052. [Google Scholar] [CrossRef]
- Gierer, J. Chemistry of delignification. 2. Reactions of lignins during bleaching. Wood Sci. Technol. 1986, 20, 1–33. [Google Scholar] [CrossRef]
- Islam, M.N.; Khan, M.A.; Zaman, M.A. Comparative Study of Water Absorption Behavior of Wood and Wood Plastic Composites of Simul Using Film Neutron Radiography. J. Macromol. Sci. Part D Rev. Polym. Process. 2005, 44, 1457–1465. [Google Scholar] [CrossRef]
- Jian, Z.; He, C.; Hui, T.; Fu, J.; Min, W.; University, N.A. Performance Comparison of Three Kinds of Plant Fibers Modified Polylactic Acid Composites. Eng. Plast. Appl. 2016. [Google Scholar]
- Cui, Y.H.; Ye-Qing, Z. Interface study on wood fiber reinforced thermoplastic composites. Fiber Compos. 2006, 1, 53–57. [Google Scholar]
- Li, X.; Wu, Y.; Xia, Z. Research Progress on Interfacial Compatibility of Plant Fibers/Biodegradable Plastic. Plast. Sci. Technol. 2009, 37, 86–89. [Google Scholar]
- Espert, A.; Vilaplana, F.; Karlsson, S. Comparison of water absorption in natural cellulosic fibres from wood and one-year crops in polypropylene composites and its influence on their mechanical properties. Compos. Part A Appl. Sci. Manuf. 2004, 35, 1267–1276. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J. Study on Hydrothermal Properties of Jute/PHBV Composites. China’s Fiber Prod. 2004, 26, 241–244. [Google Scholar]
- Su, M.; Ma, X.; Zhu, L. Structure and properties of bamboo powder/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) composites. Polym. Mater. Sci. Eng. 2016, 32, 58–62. [Google Scholar]












| Sample | α-Cellulose (%) | Hemicellulose (%) | Lignin (%) | Moisture Regained (%) |
|---|---|---|---|---|
| ULF | 57.51 ± 0.20 | 29.47 ± 0.25 | 20.45 ± 0.29 | 8.83 ± 0.3 |
| TLF | 68.29 ± 0.23 | 14.62 ± 0.14 | 16.91 ± 0.48 | 6.27 ± 0.4 |
| Sample | LF Content | Tinitial (°C) | Peak Degradation Temperature (°C) | Residual Mass Fraction (%) |
|---|---|---|---|---|
| PHBV | 0% | 313.9 | 349.4 | 0 |
| ULF/PHBV | 30% | 298.4 | 325.4 | 3.45 |
| 40% | 303.9 | 343.3 | 4.13 | |
| 50% | 304.9 | 346.5 | 2.70 | |
| 60% | 296.0 | 338.2 | 7.35 | |
| 70% | 282.9 | 335.1 | 8.81 | |
| 80% | 301.5 | 346.3 | 9.51 | |
| TLF/PHBV | 30% | 291.2 | 320.9 | 0.38 |
| 40% | 292.7 | 316.8 | 3.92 | |
| 50% | 290.6 | 314.9 | 5.32 | |
| 60% | 300.8 | 347.5 | 10.91 | |
| 70% | 288.1 | 337.8 | 6.98 | |
| 80% | 294.5 | 337.4 | 8.17 |
| Strength (MPa) | 0% | 30% | 40% | 50% | 60% | 70% | 80% |
|---|---|---|---|---|---|---|---|
| ULF/PHBV | 23.53 | 37.31 | 56.56 | 63.87 | 75.23 | 72.84 | 38.87 |
| TLF/PHBV | 23.53 | 42.55 | 64.26 | 81.77 | 90.73 | 79.50 | 72.84 |
| ULF/PHBV after immersion | 22.52 | 20.22 | 30.97 | 36.67 | 44.12 | 33.52 | 11.5 |
| TLF/PHBV after immersion | 22.52 | 23.29 | 35.65 | 47.50 | 58.55 | 37.12 | 30.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wang, L.; Chen, Y.; Luo, P.; Chen, T. Properties of Luffa Fiber Reinforced PHBV Biodegradable Composites. Polymers 2019, 11, 1765. https://doi.org/10.3390/polym11111765
Guo Y, Wang L, Chen Y, Luo P, Chen T. Properties of Luffa Fiber Reinforced PHBV Biodegradable Composites. Polymers. 2019; 11(11):1765. https://doi.org/10.3390/polym11111765
Chicago/Turabian StyleGuo, Yong, Li Wang, Yuxia Chen, Panpan Luo, and Tong Chen. 2019. "Properties of Luffa Fiber Reinforced PHBV Biodegradable Composites" Polymers 11, no. 11: 1765. https://doi.org/10.3390/polym11111765