Novel Siloxane-Modified Epoxy Resins as Promising Encapsulant for LEDs
Abstract
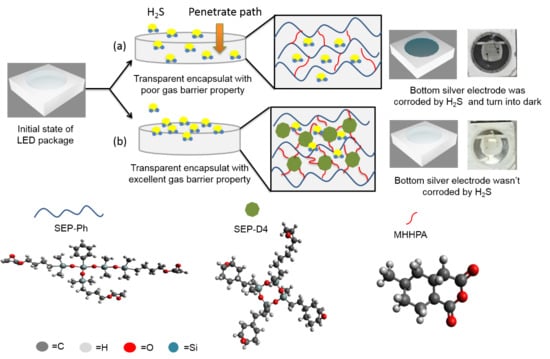
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 1,3,5,7-Tetramethyl-1,3,5,7-Tetra[(3,4-Epoxycyclohexyl)Ethyl]-Cyclotetrasiloxane (SEP-D4)
2.3. Perpetration of Transparent SEP-Ph/SEP-D4/MHHPA Compositions
2.4. Instrumentation
2.5. Encapsulation of SMD LEDs
2.6. Sulfurization Test of the Encapsulated SMD LEDs
3. Results and Discussion
3.1. Curing Behavior and Miscibility of the Transparent Siloxane-Modified Epoxy Compositions
3.2. Optical Properties and Thermal Discoloration Resistance of the Transparent Siloxane-Modified Epoxy Compositions
3.3. Thermal Decomposition and Mechanical Properties of the Transparent Siloxane-Modified Epoxy Compositions
3.4. Sulfurization Resistance of the Transparent Siloxane-Modified Epoxy Compositions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakamura, S.; Mukai, T.; Senoh, M. Candela-class high-brightness InGaN/AlGaN double-heterostructure blue-light-emitting diodes. Appl. Phys. Lett. 1994, 64, 1687–1689. [Google Scholar]
- Yam, Y.K.; Hassan, Z. Innovative advances in LED technology. Microelectron. J. 2005, 36, 129–137. [Google Scholar] [CrossRef]
- Pimputkar, S.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. Prospects for LED lighting. Nat. Photonics 2009, 3, 180–182. [Google Scholar] [CrossRef]
- Chang, M.H.; Das, D.; Varde, P.V.; Pecht, M. Light emitting diodes reliability review. Microelectron. Reliab. 2012, 52, 762–782. [Google Scholar] [CrossRef]
- Narendran, N.; Gu, Y.; Freyssinier, J.P.; Yu, H.; Deng, L. Solid-state lighting: Failure analysis of white LEDs. J. Cryst. Growth 2004, 268, 449–456. [Google Scholar] [CrossRef]
- Zibold, A.; Dammann, M.; Schmidt, R.; Konstanzer, H.; Kunzer, M. Influence of air pollutants on the lifetime of LEDs and analysis of degradation effects. Microelectron. Reliab. 2017, 77, 566–570. [Google Scholar] [CrossRef]
- Huang, J.C.; Chu, Y.P.; Wei, M.; Deanin, R.D. Comparison of epoxy resins for applications in light-emitting diodes. Adv. Polym. Technol. 2004, 23, 298–306. [Google Scholar] [CrossRef]
- Morita, Y. Cationic polymerization of hydrogenated bisphenol-A glycidyl ether with cycloaliphatic epoxy resin and its thermal discoloration. J. Appl. Polym. Sci. 2005, 97, 1395–1400. [Google Scholar] [CrossRef]
- Norris, A.W.; Bahadur, M.; Yoshitake, M. In Novel silicone materials for LED packaging. In Proceedings of the SPIE 5941, Fifth International Conference on Solid State Lighting, San Diego, CA, USA, 14 September 2005. [Google Scholar]
- Ha, S.Y.; Park, H.B.; Lee, Y.M. Percolational effect of siloxane content in poly (amideimide siloxane) on the gas permeation behavior. Macromolecules 1999, 32, 2394–2396. [Google Scholar] [CrossRef]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lee, J.L. The synthesis, characterization, and photoinitiated cationic polymerization of silicon-containing epoxy resins. J. Polym. Sci. Part A Polym. Chem. 1990, 28, 479–503. [Google Scholar] [CrossRef]
- Falk, B.; Crivello, J.V. Synthesis of epoxy-functional microspheres by cationic suspension photopolymerization. Chem. Mater. 2004, 16, 5033–5041. [Google Scholar] [CrossRef]
- Morita, Y. Curing of epoxy siloxane monomer with anhydride. J. Appl. Polym. Sci. 2005, 97, 946–951. [Google Scholar] [CrossRef]
- Morita, Y.; Tajima, S.; Suzuki, H.; Sugino, H. Thermally initiated cationic polymerization and properties of epoxy siloxane. J. Appl. Polym. Sci. 2006, 100, 2010–2019. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Yu, Y.; Yuan, Y.J. Studies on UV-stable silicone–epoxy resins. Appl. Polym. Sci. 2007, 104, 3954–3959. [Google Scholar] [CrossRef]
- Yang, S.C.; Kim, J.S.; Jin, J.H.; Kwak, S.Y.; Bae, B.S. Thermal resistance of cycloaliphatic epoxy hybrimer based on sol-gel derived oligosiloxane for LED encapsulation. J. Appl. Polym. Sci. 2010, 117, 2140–2145. [Google Scholar] [CrossRef]
- Hsu, C.W.; Ma, C.C.M.; Tan, C.S.; Li, H.T.; Huang, S.C.; Lee, T.M.; Tai, H. Effect of thermal aging on the optical, dynamic mechanical, and morphological properties of phenylmethylsiloxane-modified epoxy for use as an LED encapsulant. Mater. Chem. Phys. 2012, 134, 789–796. [Google Scholar] [CrossRef]
- Lee, S.; Hong, J.Y.; Jang, J. Multifunctional graphene sheets embedded in silicone encapsulant for superior performance of light-emitting diodes. ACS Nano 2013, 7, 5784–5790. [Google Scholar] [CrossRef]
- Topolniak, I.; Chapel, A.; Gaume, J.; Bussiere, P.O.; Chaderyon, G.; Gardette, J.L.; Therias, S. Applications of polymer nanocomposites as encapsulants for solar cells and LEDs: Impact of photodegradation on barrier and optical properties. Polym. Degrad. Stab. 2017, 145, 52–59. [Google Scholar] [CrossRef]
- Zhu, H.; Dai, Z.; Tu, W. Study on the preparation and performance of low gas permeability trifluoropropyl phenyl silicone rubber. RSC Adv. 2017, 7, 39739–39747. [Google Scholar] [CrossRef] [Green Version]
- Leterrier, Y. Durability of nanosized oxygen-barrier coatings on polymers. Prog. Mater. Sci. 2003, 48, 1–55. [Google Scholar] [CrossRef] [Green Version]
- Affinito, J.D.; Gross, M.E.; Coronado, C.A.; Graff, G.L.; Greenwell, I.N.; Martin, P.M. A new method for fabricating transparent barrier layers. Thin Solid Films 1996, 291, 63–67. [Google Scholar] [CrossRef]
- Putzien, S.; Nyuken, O.; Kühn, F.E. Functionalized polysilalkylene siloxanes (polycarbosiloxanes) by hydrosilylation—Catalysis and synthesis. Prog. Polym. Sci. 2010, 35, 687–713. [Google Scholar] [CrossRef]
- Ortiz, R.A.; Sangermano, M.; Bongiovanni, R.; Valdez, A.E.G.; Durate, L.B.; Saucedo, I.P.; Priola, A. Synthesis of hybrid methacrylate-silicone-cyclohexanepoxide monomers and the study of their UV induced polymerization. Prog. Org. Coat. 2006, 57, 159–164. [Google Scholar] [CrossRef]
- Gao, N.; Liu, W.Q.; Yan, Z.L.; Wang, Z.F. Synthesis and properties of transparent cycloaliphatic epoxy–silicone resins for opto-electronic devices packaging. Opt. Mater. 2013, 35, 567–575. [Google Scholar] [CrossRef]
- Rimdust, R.; Ishida, H. Development of new class of electronic packaging materials based on ternary systems of benzoxazine, epoxy, and phenolic resins. Polymer 2000, 41, 7941–7949. [Google Scholar] [CrossRef]
- Hu, J.; Yang, L.; Shin, M.W. Mechanism and thermal effect of delamination in light-emitting diode packages. Microelectron. J. 2007, 38, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Huang, W.; Yu, Y. Synthesis, characterization, and properties of silicone–epoxy resins. J. Appl. Polym. Sci. 2011, 120, 1216–1224. [Google Scholar] [CrossRef]
- Park, S.J.; Jin, F.L. Thermal stabilities and dynamic mechanical properties of sulfone-containing epoxy resin cured with anhydride. Polym. Degrad. Stab. 2004, 86, 515–520. [Google Scholar] [CrossRef]
- Liu, W.S.; Wang, Z.G.; Chen, Z.; Li, J.F. Synthesis and properties of two novel silicon-containing cycloaliphatic epoxy resins for electronic packaging application. Polym. Adv. Technol. 2012, 23, 367–374. [Google Scholar] [CrossRef]
- Srithawatpong, R.; Peng, Z.L.; Olson, B.G.; Jamieson, A.M.; Simha, R.; McGervey, J.D.; Maier, T.R.; Halasa, A.F.; Ishida, H. Positron annihilation lifetime studies of changes in free volume on cross-linking cis-polyisoprene, high-vinyl polybutadiene, and their miscible blends. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2754–2770. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Chung, T.S. Chemical cross-linking modification of polyimide membranes for gas separation. J. Membr. Sci. 2001, 189, 231–239. [Google Scholar] [CrossRef]
- Tin, P.S.; Chung, T.S.; Liu, Y.; Wang, R.; Liu, S.L.; Pramod, K.P. Effects of cross-linking modification on gas separation performance of Matrimid membranes. J. Membr. Sci. 2003, 225, 77–90. [Google Scholar] [CrossRef]







| Sample ID | Sample 1 | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|---|
| Compound | SEP-Ph | SEP-Ph/SEP-D4 = 0.8/0.2 | SEP-Ph/SEP-D4 = 0.5/0.5 | SEP-D4 |
| SEP-Ph | 1 | 0.8 | 0.5 | 0 |
| SEP-D4 | 0 | 0.2 | 0.5 | 1 |
| MHHPA | 1 | 1 | 1 | 1 |
| Sample | T (K) | E′ @T (MPa) | ρ(10−3 mol/cm3) |
|---|---|---|---|
| Sample 1 | 340.15 | 15.52 | 1.83 |
| Sample 2 | 350.15 | 18.22 | 2.00 |
| Sample 3 | 384.15 | 49.11 | 5.13 |
| Sample 4 | 523.15 | 97.76 | 7.49 |
| Sample | Luminous Flux Before Sulfur Test (lm) | Luminous Flux After Sulfur Test for 240 h (lm) | Luminous Flux Retention Percentage (%) |
|---|---|---|---|
| Sample 1 | 6.6 | 5.5 | 83.3 |
| Sample 2 | 6.6 | 5.7 | 86.4 |
| Sample 3 | 6.6 | 6.5 | 98.5 |
| Sample 4 | 6.8 | 6.6 | 97.1 |
| Commercial silicone | 6.7 | 4.9 * | 73.1 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Whang, W.-T.; Chen, C.-H.; Huang, S.-C.; Chen, K.-C. Novel Siloxane-Modified Epoxy Resins as Promising Encapsulant for LEDs. Polymers 2020, 12, 21. https://doi.org/10.3390/polym12010021
Lin C-H, Whang W-T, Chen C-H, Huang S-C, Chen K-C. Novel Siloxane-Modified Epoxy Resins as Promising Encapsulant for LEDs. Polymers. 2020; 12(1):21. https://doi.org/10.3390/polym12010021
Chicago/Turabian StyleLin, Chih-Hao, Wha-Tzong Whang, Chun-Hua Chen, Shu-Chen Huang, and Kai-Chi Chen. 2020. "Novel Siloxane-Modified Epoxy Resins as Promising Encapsulant for LEDs" Polymers 12, no. 1: 21. https://doi.org/10.3390/polym12010021