Immobilization of Trypsin from Porcine Pancreas onto Chitosan Nonwoven by Covalent Bonding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Method
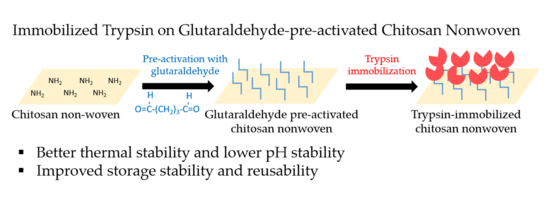
2.2.1. Preparation of Pre-Activated Chitosan Nonwoven with Glutaraldehyde
2.2.2. Immobilization of Trypsin onto Glutaraldehyde-Pre-Activated Chitosan Nonwoven
2.2.3. Immobilized-Trypsin Activity Assay
2.2.4. pH and Thermal Stability of Free and Immobilized Enzyme
2.2.5. Storage Stability of Immobilized Trypsin
2.2.6. Reusability of Immobilized Trypsin
2.2.7. Characterization of Trypsin Immobilization
3. Results and Discussion
3.1. Effects of pH, Trypsin Concentration, and Immobilization Time on Trypsin Immobilization
3.2. Evaluation of the Stability of Free and Immobilized Trypsin
3.3. Surface Observation of Trypsin-Immobilized Nonwoven Fabric
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guedidi, S.; Yurekli, Y.; Deratani, A.; Déjardin, P.; Innocent, C.; Altinkaya, S.A.; Roudesli, S.; Yemenicioglu, A. Effect of enzyme location on activity and stability of trypsin and urease immobilized on porous membranes by using layer-by-layer self-assembly of polyelectrolyte. J. Membr. Sci. 2010, 365, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; He, B.-H.; Zhao, G.-L.; Qian, L.-Y.; Li, X.-F. Immobilization of pectinase on oxidized pulp fiber and its application in whitewater treatment. Carbohydr. Polym. 2013, 97, 523–529. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Kim, M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar]
- Kim, H.R.; Seo, H.Y. Enzymatic hydrolysis of polyamide fabric by using acylase. Text. Res. J. 2013, 83, 1181–1189. [Google Scholar] [CrossRef]
- Kim, H.R.; Song, W.S. Lipase treatment of polyester fabrics. Fibers Polym. 2006, 7, 339–343. [Google Scholar] [CrossRef]
- Lee, S.H.; Song, W.S. Surface modification of polyester fabrics by enzyme treatment. Fibers Polym. 2010, 11, 54–59. [Google Scholar] [CrossRef]
- Lee, S.H.; Song, W.S. Modification of polylactic acid fabric by two lipolytic enzyme hydrolysis. Text. Res. J. 2013, 83, 229–237. [Google Scholar] [CrossRef]
- Song, A.R.; Kim, H.R.; Song, W.S. Optimization of enzymatic treatment of polyamide fabrics by bromelain. Fibers Polym. 2012, 13, 282–288. [Google Scholar] [CrossRef]
- Cristóvão, R.O.; Silvério, S.C.; Tavares, A.P.M.; Brígida, A.I.S.; Loureiro, J.M.; Boaventura, R.A.R.; Macedo, E.A.; Coelho, M.A.Z. Green coconut fiber: A novel carrier for the immobilization of commercial laccase by covalent attachment for textile dyes decolourization. World J. Microbiol. Biotechnol. 2012, 28, 2827–2838. [Google Scholar] [CrossRef]
- Guerrero, E.; Aburto, P.; Terrés, E.; Villegas, O.; González, E.; Zayas, T.; Hernández, F.; Torres, E. Improvement of catalytic efficiency of chloroperoxidase by its covalent immobilization on SBA-15 for azo dye oxidation. J. Porous Mater. 2013, 20, 387–396. [Google Scholar] [CrossRef]
- Pazarlioğlu, N.K.; Sariişik, M.; Telefoncu, A. Treating denim fabrics with immobilized commercial cellulases. Process Biochem. 2005, 40, 767–771. [Google Scholar] [CrossRef]
- Patra, A.K.; Madhu, A.; Bala, N. Enzyme washing of indigo and sulphur dyed denim. Fash. Text. 2018, 5, 3. [Google Scholar] [CrossRef]
- Khanna, S.; Chakraborty, J.N. Optimization of monochlorotriazine β-cyclodextrin grafting on cotton and assessment of release behavior of essential oils from functionalized fabric. Fash. Text. 2017, 4, 6. [Google Scholar] [CrossRef]
- Silva, C.; Silva, C.J.; Zille, A.; Guebitz, G.M.; Cavaco-Paulo, A. Laccase immobilization on enzymatically functionalized polyamide 6,6 fibres. Enzym. Microb. Technol. 2007, 41, 867–875. [Google Scholar] [CrossRef] [Green Version]
- Soleimani, M.; Khani, A.; Najafzadeh, K. α-Amylase immobilization on the silica nanoparticles for cleaning performance towards starch soils in laundry detergents. J. Mol. Catal. B Enzym. 2012, 74, 1–5. [Google Scholar] [CrossRef]
- Wang, Q.; Fan, X.; Hu, Y.; Yuan, J.; Cui, L.; Wang, P. Antibacterial functionalization of wool fabric via immobilizing lysozymes. Bioprocess Biosyst. Eng. 2009, 32, 633–639. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Chellapandian, M.; Sastry, C.A. Immobilization of alkaline protease on nylon. Bioprocess Eng. 1994, 11, 17–21. [Google Scholar] [CrossRef]
- Romdhane, I.B.-B.; Romdhane, Z.B.; Gargouri, A.; Belghith, H. Esterification activity and stability of Talaromyces thermophilus lipase immobilized onto chitosan. J. Mol. Catal. B Enzym. 2011, 68, 230–239. [Google Scholar] [CrossRef]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Enzyme Immobilization on Chitin and Chitosan-Based Supports for Biotechnological Applications. In Sustainable Agriculture Reviews 35: Chitin and Chitosan: History, Fundamentals and Innovations; Crini, G., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2019; pp. 147–173. [Google Scholar]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Dinçer, A.; Becerik, S.; Aydemir, T. Immobilization of tyrosinase on chitosan–clay composite beads. Int. J. Biol. Macromol. 2012, 50, 815–820. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Aly, A.S.; Mohamed, T.M.; Salah, H.A. Immobilization of Horseradish Peroxidase on Nonwoven Polyester Fabric Coated with Chitosan. Appl. Biochem. Biotechnol. 2008, 144, 169–179. [Google Scholar] [CrossRef]
- Orrego, C.E.; Salgado, N.; Valencia, J.S.; Giraldo, G.I.; Giraldo, O.H.; Cardona, C.A. Novel chitosan membranes as support for lipases immobilization: Characterization aspects. Carbohydr. Polym. 2010, 79, 9–16. [Google Scholar] [CrossRef]
- Xi, F.; Wu, J.; Jia, Z.; Lin, X. Preparation and characterization of trypsin immobilized on silica gel supported macroporous chitosan bead. Process Biochem. 2005, 40, 2833–2840. [Google Scholar] [CrossRef]
- Li, W.; Chen, B.; Tan, T. Comparative study of the properties of lipase immobilized on nonwoven fabric membranes by six methods. Process Biochem. 2011, 46, 1358–1365. [Google Scholar] [CrossRef]
- Bösiger, P.; Tegl, G.; Richard, I.M.T.; Le Gat, L.; Huber, L.; Stagl, V.; Mensah, A.; Guebitz, G.M.; Rossi, R.M.; Fortunato, G. Enzyme functionalized electrospun chitosan mats for antimicrobial treatment. Carbohydr. Polym. 2018, 181, 551–559. [Google Scholar] [CrossRef]
- Cheng, F.; Gao, J.; Wang, L.; Hu, X. Composite chitosan/poly (ethylene oxide) electrospun nanofibrous mats as novel wound dressing matrixes for the controlled release of drugs. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rejeena, S.R. Adsorption and hydrolytic activity of trypsin on a carboxylate-functionalized cation exchanger prepared from nanocellulose. J. Colloid Interface Sci. 2012, 381, 125–136. [Google Scholar] [CrossRef]
- Nikolic, T.; Kostic, M.; Praskalo, J.; Pejic, B.; Petronijevic, Z.; Skundric, P. Sodium periodate oxidized cotton yarn as carrier for immobilization of trypsin. Carbohydr. Polym. 2010, 82, 976–981. [Google Scholar] [CrossRef]
- Dartiguenave, C.; Hamad, H.; Waldron, K.C. Immobilization of trypsin onto 1,4-diisothiocyanatobenzene-activated porous glass for microreactor-based peptide mapping by capillary electrophoresis: Effect of calcium ions on the immobilization procedure. Anal. Chim. Acta 2010, 663, 198–205. [Google Scholar] [CrossRef]
- Peng, G.; Zhao, C.; Liu, B.; Ye, F.; Jiang, H. Immobilized trypsin onto chitosan modified monodisperse microspheres: A different way for improving carrier’s surface biocompatibility. Appl. Surf. Sci. 2012, 258, 5543–5552. [Google Scholar] [CrossRef]
- Manrich, A.; Galvão, C.M.A.; Jesus, C.D.F.; Giordano, R.C.; Giordano, R.L.C. Immobilization of trypsin on chitosan gels: Use of different activation protocols and comparison with other supports. Int. J. Biol. Macromol. 2008, 43, 54–61. [Google Scholar] [CrossRef]
- Monteiro, F.M.F.; de Medeiros e Silva, G.M.; da Silva, J.B.R.; Porto, C.S.; de Carvalho, L.B.; de Lima Filho, J.L.; Carneiro-Leão, A.M.D.A.; Carneiro-da-Cunha, M.D.G.; Porto, A.L.F. Immobilization of trypsin on polysaccharide film from Anacardium occidentale L. and its application as cutaneous dressing. Process Biochem. 2007, 42, 884–888. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S.H.; Song, W.S. Immobilization of Trypsin on Chitosan Nonwoven Using Glutaraldehyde. J. Korean Soc. Cloth. Text. 2013, 37, 852–863. [Google Scholar] [CrossRef] [Green Version]
- Monsan, P. Optimization of glutaraldehyde activation of a support for enzyme immobilization. J. Mol. Catal. 1978, 3, 371–384. [Google Scholar] [CrossRef]
- Rehman, H.U.; Aman, A.; Silipo, A.; Qader, S.A.U.; Molinaro, A.; Ansari, A. Degradation of complex carbohydrate: Immobilization of pectinase from Bacillus licheniformis KIBGE-IB21 using calcium alginate as a support. Food Chem. 2013, 139, 1081–1086. [Google Scholar] [CrossRef]
- Li, F.-Y.; Xing, Y.-J.; Ding, X. Immobilization of papain on cotton fabric by sol–gel method. Enzym. Microb. Technol. 2007, 40, 1692–1697. [Google Scholar] [CrossRef]
- Monier, M.; El-Sokkary, A.M.A. Modification and characterization of cellulosic cotton fibers for efficient immobilization of urease. Int. J. Biol. Macromol. 2012, 51, 18–24. [Google Scholar] [CrossRef]
- Zelenetskii, A.N.; Akopova, T.A.; Kildeeva, N.R.; Vikhoreva, G.A.; Obolonkova, E.S.; Zharov, A.A. Immobilization of trypsin on polysaccharides upon intense mechanical treatment. Russ. Chem. Bull. 2003, 52, 2073–2077. [Google Scholar] [CrossRef]










| Composition (%) | Thickness (mm) | Weight (g/m2) | Manufacturing Method | Type |
|---|---|---|---|---|
| Chitosan 100 | 0.38 | 88.5 | Needle punching | Spunlace |
| Enzyme | Source | Activity | Form | Manufacturer |
|---|---|---|---|---|
| Trypsin (EC 3.4.21.4) | Porcine pancreas | 1000–2000 BAEE units/mg | Lyphophilized powder | Sigma Chemicals Co. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.S.; Lee, S. Immobilization of Trypsin from Porcine Pancreas onto Chitosan Nonwoven by Covalent Bonding. Polymers 2019, 11, 1462. https://doi.org/10.3390/polym11091462
Kim JS, Lee S. Immobilization of Trypsin from Porcine Pancreas onto Chitosan Nonwoven by Covalent Bonding. Polymers. 2019; 11(9):1462. https://doi.org/10.3390/polym11091462
Chicago/Turabian StyleKim, Jung Soo, and Sohee Lee. 2019. "Immobilization of Trypsin from Porcine Pancreas onto Chitosan Nonwoven by Covalent Bonding" Polymers 11, no. 9: 1462. https://doi.org/10.3390/polym11091462