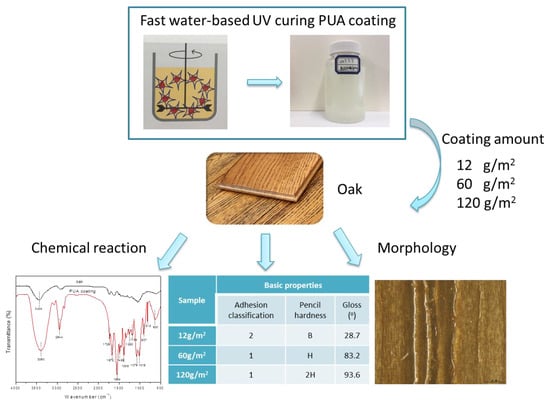
Preparation of a Fast Water-Based UV Cured Polyurethane-Acrylate Wood Coating and the Effect of Coating Amount on the Surface Properties of Oak (Quercus alba L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Fast Water-Based UV Curing PUA Coating
2.3. Coating on a Wood Sample
2.4. Characterizations and Tests
3. Results and Discussion
3.1. Morphologies
3.2. Basic Properties of PUA Coated Oak
3.3. Chemical Analysis
3.4. Contact Angle
3.5. Surface Roughness
3.6. Penetration of PUA in Wood
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, H.; Qiu, F.; Wang, Y.; Wu, W.; Yang, D.; Guo, Q. UV-curable waterborne polyurethane-acrylate: Preparation, characterization and properties. Prog. Org. Coat. 2012, 73, 47–53. [Google Scholar] [CrossRef]
- Qi, L.; Guo, L.; Teng, Q.; Xiao, W.; Du, D.; Li, X. Synthesis of waterborne polyurethane containing alkoxysilane side groups and the properties of the hybrid coating films. Appl Surf. Sci. 2016, 377, 66–74. [Google Scholar]
- Chang, C.W.; Lu, K.T. Epoxy acrylate UV/PU dual-cured wood cotings. J. Appl. Polym. Sci. 2012, 115, 2197–2202. [Google Scholar] [CrossRef]
- Meijer, M.D. Review on the durability of exterior wood coatings with reduced VOC-content. Prog. Org. Coat. 2001, 43, 217–225. [Google Scholar] [CrossRef]
- Chang, W.Y.; Pan, Y.W.; Chuang, C.N.; Guo, J.J.; Chen, S.H.; Wang, C.K.; Hsieh, K.H. Fabrication and characterization of waterborne polyurethane (WPU) with aluminum trihydroxide (ATH) and mica as flame retardants. J. Polym. Res. 2015, 22, 243. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, H.; Liu, Y.; Li, J.; Lu, Y.; Hunt, J.F. Improvement of water resistance and dimensional stability of wood through titanium dioxide coating. Holzforschung 2010, 64, 757–761. [Google Scholar] [CrossRef]
- Xu, H.; Qiu, F.; Wang, Y.; Yang, D.; Wu, W.; Chen, Z.; Zhu, J. Preparation, mechanical properties of waterborne polyurethane and crosslinked polyurethane-acrylate composite. J. Appl. Polym. Sci. 2012, 124, 958–968. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, F.; Xu, B.; Xu, J.; Jiang, Y.; Yang, D.; Lia, P. Preparation, mechanical properties and surface morphologies of waterborne fluorinated polyurethane-acrylate. Prog. Org. Coat. 2013, 76, 876–883. [Google Scholar] [CrossRef]
- Chou, P.L.; Chang, H.T.; Yeh, T.F.; Chang, S.T. Characterizing the conservation effect of clear coatings on photodegradation of wood. Bioresource Technol. 2008, 99, 1073–1079. [Google Scholar] [CrossRef]
- Liu, F.; Liu, G. Enhancement of UV-aging resistance of UV-curable polyurethane acrylate coatings via incorporation of hindered amine light stabilizers-functionalized TiO2-SiO2 nanoparticles. J. Polym. Res. 2018, 25, 59. [Google Scholar] [CrossRef]
- Decker, C.; Masson, F.; Schewalm, R. How to speed up the UV curing of water-based acrylic coatings. JCT Res. 2004, 1, 127–136. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Liu, X.; Han, L.; Wu, Y.; Dai, X.; Zhu, J. Synthesis of bio-based unsaturated polyester resins and their application in waterborne UV-curable coatings. Prog. Org. Coat. 2015, 78, 49–54. [Google Scholar] [CrossRef]
- Montazeri, M.; Eckelman, M.J. Life cycle assessment of UV-Curable bio-based wood flooring coatings. J. Clean. Prod. 2018, 192, 932–939. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Y.; Zhang, T.; Dai, Y.; Yang, D.; Qiu, F.; Yu, Z.; Yang, P. Synthesis of UV-curing waterborne polyurethane-acrylate coating and its photopolymerization kinetics using FT-IR and photo-DSC methods. Prog. Org. Coat. 2018, 122, 10–18. [Google Scholar] [CrossRef]
- Decker, C.; Masson, F.; Schwalm, R. Weathering resistance of waterbased UV-cured polyurethane-acrylate coatings. Polym. Degrad. Stabil. 2004, 83, 309–320. [Google Scholar] [CrossRef]
- Masson, F.; Decker, C.; Jaworek, T.; Schwalm, R. UV-radiation curing of waterbased urethane-acrylate coatings. Prog. Org. Coat. 2000, 39, 115–126. [Google Scholar] [CrossRef]
- Sonmez, A.; Budakci, M.; Bayram, M. Effect of wood moisture content on adhesion of varnish coatings. Sci. Res. Essays. 2010, 12, 1432–1437. [Google Scholar]
- Brown, G.L. Formation of films from polymer dispersions. J. Polym. Sci. A 1956, 102, 423–434. [Google Scholar] [CrossRef]
- Hwang, H.D.; Park, C.H.; Moon, J.I.; Kim, H.J.; Masubuchi, T. UV-curing behavior and physical properties of waterborne UV-curable polycarbonate-based polyurethane dispersion. Prog. Org. Coat. 2011, 72, 663–675. [Google Scholar] [CrossRef]
- Hahn, K.; Ley, G.; Schuller, H.; Oberthür, R. On particle coalescence in latex films. Colloid. Polym. Sci. 1986, 264, 1092–1096. [Google Scholar] [CrossRef]
- Tong, T.; Lu, Z.; Zhou, G.; Jia, W.; Wang, M. Effect of air velocity in dehumidification drying environment on one-component waterborne wood top coating drying process. Dry. Technol. 2016, 34, 7372015–7373937. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Wu, Y.; Liu, J.; Cheng, F.; Jiao, X.; Lai, G. Fabrication of UV-curable solvent-free epoxy modified silicone resin coating with high transparency and low volume shrinkage. Prog. Org. Coat. 2019, 129, 96–100. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, L.; Han, D.; Li, W.; Chen, Y.; Wang, X.; Ning, L. Preparation and hydrophobicity failure behavior of two kinds of fluorine-containing acrylic polyurethane coatings. Rsc Adv. 2015, 115, 95230–95239. [Google Scholar] [CrossRef]
- Kong, X.; Qu, J.; Zhu, Y.; Cao, S.; Chen, H. Adhesion behaviors and affecting factors of polymer latexes. Paint. Coat. Ind. 2010, 40, 37–40. [Google Scholar]
- Chu, H.H.; Chang, C.Y.; Shen, B.H. An electrophoretic coating using a nanosilica modified polyacrylate resin. J. Polym. Res. 2018, 25, 44. [Google Scholar] [CrossRef]
- Asif, A.; Shi, W.; Shen, X.; Nie, K. Physical and thermal properties of UV curable waterborne polyurethane dispersions incorporating hyperbranched aliphatic polyester of varying generation number. Polymer 2005, 46, 11066–11078. [Google Scholar] [CrossRef]
- Asif, A.; Shi, W. UV curable waterborne polyurethane acrylate dispersions based on hyperbranched aliphatic polyester: Effect of molecular structure on physical and thermal properties. Polym. Adv. Technol. 2004, 15, 669–675. [Google Scholar] [CrossRef]
- Asif, A.; Huang, C.Y.; Shi, W.F. Photopolymerization of waterborne polyurethane acrylate dispersions based on hyperbranched aliphatic polyester and properties of the cured films. Colloid. Polym. Sci. 2005, 283, 721–730. [Google Scholar] [CrossRef]
- Asif, A.; Hu, L.; Shi, W. Synthesis, rheological, and thermal properties of waterborne hyperbranched polyurethane acrylate dispersions for UV curable coatings. Colloid. Polym. Sci. 2009, 287, 1041–1049. [Google Scholar] [CrossRef]
- Phelps, J.E.; Workman, E.C., Jr. Vessel area studies in white oak (Quercus alba L.). Wood Fiber Sci. 1994, 26, 315–322. [Google Scholar]
- Ozyhar, T.; Mohl, L.; Hering, S.; Hass, P.; Zeindler, L.; Ackermann, R.; Niemz, P. Orthotropic hygric and mechanical material properties of oak wood. Wood Mater. Sci. Eng. 2016, 11, 36–45. [Google Scholar] [CrossRef]
- Catalin, C.; Cosmin, S.; Aurel, L.; Daniel, C.; Claudiu, R.I.; Alin, P.M.; Tibor, B.; Manuela, S.E.; Alexandru, P. Surface properties of thermally treated composite wood panels. Appl. Surf. Sci. 2018, 438, 114–126. [Google Scholar]
- Xu, J.; Liu, R.; Wu, H.; Long, L.; Lin, P. A comparison of the performance of two kinds of waterborne coatings on bamboo and bamboo scrimber. Coatings 2019, 9, 161. [Google Scholar] [CrossRef]
- Kuang, X.; Kuang, R.; Zheng, X.; Wang, Z. Mechanical properties and size stability of wheat straw and recycled LDPE composites coupled by waterborne coupling agents. Carbohyd. Polym. 2010, 80, 927–933. [Google Scholar] [CrossRef]
- Landry, V.; Blanchet, P. Surface preparation of wood for application of waterborne coatings. For. Prod. J. 2012, 62, 39–45. [Google Scholar] [CrossRef]










| Reagent | Function | Manufacturer |
|---|---|---|
| Ammonium persulfate (APS) | Initiator | 1 |
| Acrylic acid (AA) | Monomer | 1 |
| Styrene (ST) | Monomer | 1 |
| Ethyl acrylate (EA) | Monomer | 1 |
| Hydroxyethyl acrylate (HEA) | Monomer | 1 |
| Toluene-2,4-diisocyanate (TDI) | Monomer | 1 |
| Polyethylene glycol (Mn: 400) (PEG-400) | Monomer | 1 |
| Dibutyltin dilaurate (DBTD) | Catalyst | 1 |
| N-methyl-2-pyrrolidone | Solvent | 1 |
| Hexanediol diacrylate (HDDA) | Diluting agent | 1 |
| γ-Aminopropyl triethoxysilane (KH550) | Silane | 2 |
| Polyether siloxane copolymer composition | Defoamer agent | 3 |
| 2-Hydroxymethylphenylpropan-1-one | Photoinitiator | 4 |
| Parameter | Values |
|---|---|
| Solid content | 90 wt% |
| Viscosity | 50 mpa·s |
| Average particle size | 226 nm |
| Technical requirements | Standards | Adhesion classification | Pencil hardness | Curing time (min) |
|---|---|---|---|---|
| Water-based coatings for woodenware for indoor decorating and refurbishing | GB/T 23999-2009 | ≤1 | ≥B | 30–60 |
| EN 927:2006 | ≤1 | -- | -- | |
| UV curing coatings for woodenware | HG/T 3655–2012 | ≤2 | ≥H | -- |
| Wooden furniture | GB/T 3324–2017 | ≤3 | -- | -- |
| CEN/TS 16209:2011 | ≤2 | -- | -- | |
| Solid wood floor | GB/T 15036–2018 | ≤3 | ≥H | -- |
| ISO 17959:2014 | ≤3 | ≥H | -- | |
| Engineered wood floor | GB/T 18103–2013 | ≤2 | ≥2H | -- |
| EN 14354–2005 | ≤2 | -- | -- | |
| Indoor wood-based door | LY/T 1923–2010 | ≤2 | ≥HB | -- |
| Wood-based wall-board | LY/T 1697–2017 | ≤2 | ≥2B | -- |
| Sample | Dried coating thickness (μm) | Adhesion classification | Pencil hardness | Gloss (°) |
|---|---|---|---|---|
| 12 g/m2 PUA coated oak | 3 | 2 | B | 28.7 |
| 60 g/m2 PUA coated oak | 34 | 1 | H | 83.2 |
| 120 g/m2 PUA coated oak | 74 | 1 | 2H | 93.6 |
| Sample | Testing liquid | Contact angle (°) |
|---|---|---|
| Pure oak | Water | 29.68 |
| Pure oak | 70 wt% PUA droplet | 34.98 |
| 12g/m2 PUA coated oak | Water | 52.22 |
| 60g/m2 PUA coated oak | Water | 56.13 |
| Sample | Ra (μm) | Rp (μm) | Rv (μm) | Rt (μm) | Rz (μm) |
|---|---|---|---|---|---|
| Pure oak | 3.456 | 5.930 | 12.521 | 55.110 | 18.450 |
| 12 g/m2 PUA coated oak | 3.026 | 3.451 | 10.000 | 45.975 | 13.451 |
| 60 g/m2 PUA coated oak | 2.422 | 1.651 | 6.924 | 48.010 | 8.577 |
| 120 g/m2 PUA coated oak | 0.310 | 0.516 | 0.400 | 2.834 | 0.916 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wu, H.; Liu, R.; Long, L.; Xu, J.; Chen, M.; Qiu, H. Preparation of a Fast Water-Based UV Cured Polyurethane-Acrylate Wood Coating and the Effect of Coating Amount on the Surface Properties of Oak (Quercus alba L.). Polymers 2019, 11, 1414. https://doi.org/10.3390/polym11091414
Wang J, Wu H, Liu R, Long L, Xu J, Chen M, Qiu H. Preparation of a Fast Water-Based UV Cured Polyurethane-Acrylate Wood Coating and the Effect of Coating Amount on the Surface Properties of Oak (Quercus alba L.). Polymers. 2019; 11(9):1414. https://doi.org/10.3390/polym11091414
Chicago/Turabian StyleWang, Jin, Huagui Wu, Ru Liu, Ling Long, Jianfeng Xu, Minggui Chen, and Hongyun Qiu. 2019. "Preparation of a Fast Water-Based UV Cured Polyurethane-Acrylate Wood Coating and the Effect of Coating Amount on the Surface Properties of Oak (Quercus alba L.)" Polymers 11, no. 9: 1414. https://doi.org/10.3390/polym11091414