Preparation and Characterization of PVA/PDDA/Nano-Zirconia Composite Anion Exchange Membranes for Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
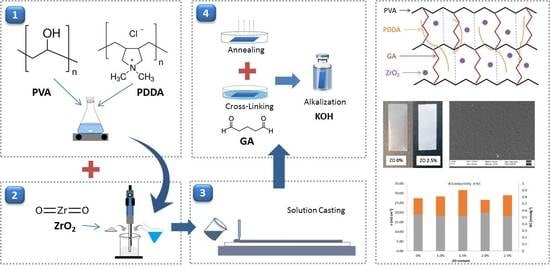
2.1. Membrane Preparation
2.2. Cross-Linking
2.3. Structure Characterization
2.4. Swelling Properties
2.5. Ion Exchange Capacity (IEC)
2.6. OH− Conductivity
3. Results and Discussion
3.1. Chemical Structure
3.2. Morphology
3.3. Thermal Analysis
3.4. Mechanical Properties
3.5. Water Uptake and Swelling Degree
3.6. Ion-Exchange Capacity and OH− Conductivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, W.; Wang, H.; Yuan, X.; Martin, J.J.; Yang, D. A review on water balance in the membrane electrode assembly of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 9461–9478. [Google Scholar] [CrossRef]
- Fang, J.; Wu, Y.; Zhang, Y.; Lyu, M.; Zhao, J. Novel anion exchange membranes based on pyridinium groups and fluoroacrylate for alkaline anion exchange membrane fuel cells. Int. J. Hydrogen Energy 2015, 40, 12392–12399. [Google Scholar] [CrossRef]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Ameduri, B. Polymeric materials as anion-exchange membranes for alkaline fuel cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Qiao, J.; Baker, R.; Zhang, J. Alkaline polymer electrolyte membranes for fuel cell applications. Chem. Soc. Rev. 2013, 42, 5768–5787. [Google Scholar] [CrossRef] [PubMed]
- Iravaninia, M.; Rowshanzamir, S. Polysulfone-based Anion Exchange Membranes for Potential Application in Solid Alkaline Fuel Cells. J. Renew. Energy Environ. 2015, 2, 59–65. [Google Scholar]
- Hu, B.; Miao, L.; Zhao, Y.; Lü, C. Azide-assisted crosslinked quaternized polysulfone with reduced graphene oxide for highly stable anion exchange membranes. J. Memb. Sci. 2017, 530, 84–94. [Google Scholar] [CrossRef]
- Xue, J.; Liu, L.; Liao, J.; Shen, Y.; Li, N. UV-crosslinking of polystyrene anion exchange membranes by azidated macromolecular crosslinker for alkaline fuel cells. J. Memb. Sci. 2017, 535, 322–330. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Liao, J.; Wang, L.; Li, N. Self-crosslinking of comb-shaped polystyrene anion exchange membranes for alkaline fuel cell application. J. Memb. Sci. 2017, 536, 133–140. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Q.L.; Zhang, Q.G.; Zhu, A.M. Synthesis and characterization of cross-linked quaternized poly(vinyl alcohol)/chitosan composite anion exchange membranes for fuel cells. J. Power Sources 2008, 183, 447–453. [Google Scholar] [CrossRef]
- Gong, Y.; Liao, X.; Xu, J.; Chen, D.; Zhang, H. Novel anion-conducting interpenetrating polymer network of quaternized polysulfone and poly(vinyl alcohol) for alkaline fuel cells. Int. J. Hydrogen Energy 2016, 41, 5816–5823. [Google Scholar] [CrossRef]
- Ouadah, A.; Xu, H.; Luo, T.; Gao, S.; Wang, X.; Fang, Z.; Jing, C.; Zhu, C. A series of poly(butylimidazolium) ionic liquid functionalized copolymers for anion exchange membranes. J. Power Sources 2017, 371, 77–85. [Google Scholar] [CrossRef]
- Hu, Q.; Shang, Y.; Wang, Y.; Xu, M.; Wang, S.; Xie, X.; Liu, Y.; Zhang, H.; Wang, J.; Mao, Z. Preparation and characterization of fluorinated poly(aryl ether oxadiazole)s anion exchange membranes based on imidazolium salts. Int. J. Hydrogen Energy 2012, 37, 12659–12665. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, P.; Zhang, X.; Weng, Q.; Chen, X.; An, Z. Synthesis and properties of poly(arylene ether sulfone) anion exchange membranes with pendant benzyl-quaternary ammonium groups. Polym. (United Kingdom) 2017, 121, 137–148. [Google Scholar] [CrossRef]
- Hu, E.N.; Lin, C.X.; Liu, F.H.; Wang, X.Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Poly(arylene ether nitrile) anion exchange membranes with dense flexible ionic side chain for fuel cells. J. Memb. Sci. 2018, 550, 254–265. [Google Scholar] [CrossRef]
- Xu, J.; Liu, B.; Luo, X.; Li, M.; Zang, H.; Zhang, H.; Wang, Z. Construction of ion transport channels by grafting flexible alkyl imidazolium chain into functional poly(arylene ether ketone sulfone) as anion exchange membranes. Int. J. Hydrogen Energy 2017, 42, 25996–26006. [Google Scholar] [CrossRef]
- Omasta, T.J.; Wang, L.; Peng, X.; Lewis, C.A.; Varcoe, J.R.; Mustain, W.E. Importance of balancing membrane and electrode water in anion exchange membrane fuel cells. J. Power Sources 2017. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.; Zhang, F. A mini-review on anion exchange membranes for fuel cell applications: Stability issue and addressing strategies. Int. J. Hydrogen Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Li, N.; Yan, T.; Li, Z.; Thurn-Albrecht, T.; Binder, W.H. Comb-shaped polymers to enhance hydroxide transport in anion exchange membranes. Energy Environ. Sci. 2012, 5, 7888–7892. [Google Scholar] [CrossRef]
- Dang, H.S.; Weiber, E.A.; Jannasch, P. Poly(phenylene oxide) functionalized with quaternary ammonium groups via flexible alkyl spacers for high-performance anion exchange membranes. J. Mater. Chem. A 2015, 3, 5280–5284. [Google Scholar] [CrossRef] [Green Version]
- Nuñez, S.A.; Capparelli, C.; Hickner, M.A. N-Alkyl Interstitial Spacers and Terminal Pendants Influence the Alkaline Stability of Tetraalkylammonium Cations for Anion Exchange Membrane Fuel Cells. Chem. Mater. 2016, 28, 2589–2598. [Google Scholar] [CrossRef]
- Wang, C.; Shen, B.; Xu, C.; Zhao, X.; Li, J. Side-chain-type poly(arylene ether sulfone)s containing multiple quaternary ammonium groups as anion exchange membranes. J. Memb. Sci. 2015, 492, 281–288. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Ma, C.; Liu, Y.; Qiao, J. Poly (vinyl alcohol)/sulfosuccinic acid (PVA/SSA) as proton-conducting membranes for fuel cells: Effect of cross-linking and plasticizer addition. ECS Trans. 2013, 53, 29–34. [Google Scholar] [CrossRef]
- Zakeri, M.; Abouzari-Lotf, E.; Nasef, M.M.; Ahmad, A.; Ripin, A.; Ting, T.M.; Sithambaranathan, P. Preparation and characterization of highly stable protic-ionic-liquid membranes. Int. J. Hydrogen Energy 2018, 1–11. [Google Scholar] [CrossRef]
- Vinodh, R.; Sangeetha, D. Efficient utilization of anion exchange composites using silica filler for low temperature alkaline membrane fuel cells. Int. J. Plast. Technol. 2013, 17, 35–50. [Google Scholar] [CrossRef]
- Yang, C.C.; Chiu, S.J.; Chien, W.C.; Chiu, S.S. Quaternized poly(vinyl alcohol)/alumina composite polymer membranes for alkaline direct methanol fuel cells. J. Power Sources 2010, 195, 2212–2219. [Google Scholar] [CrossRef]
- Derbali, Z.; Fahs, A.; Chailan, J.F.; Ferrari, I.V.; Di Vona, M.L.; Knauth, P. Composite anion exchange membranes with functionalized hydrophilic or hydrophobic titanium dioxide. Int. J. Hydrogen Energy 2017, 42, 19178–19189. [Google Scholar] [CrossRef]
- Li, X.; Tao, J.; Nie, G.; Wang, L.; Li, L.; Liao, S. Cross-linked multiblock copoly(arylene ether sulfone) ionomer/nano-ZrO2 composite anion exchange membranes for alkaline fuel cells. RSC Adv. 2014, 4, 41398–41410. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, M.; He, X.; Qiao, J. Poly(vinyl alcohol)/Poly(diallyldimethylammonium chloride) anion-exchange membrane modified with multiwalled carbon nanotubes for alkaline fuel cells. J. Mater. 2019, 5, 286–295. [Google Scholar] [CrossRef]
- Vinodh, R.; Purushothaman, M.; Sangeetha, D. Novel quaternized polysulfone/ZrO2 composite membranes for solid alkaline fuel cell applications. Int. J. Hydrogen Energy 2011, 36, 7291–7302. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Meng, Y. Novel quaternized poly(arylene ether sulfone)/nano-ZrO2 composite anion exchange membranes for alkaline fuel cells. ACS Appl. Mater. Interfaces 2013, 5, 1414–1422. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, J.; Jingfu, J.; Jiang, G.; Zhang, J.; Qiao, J. Poly(ethylene glycol) plasticized poly(vinyl alcohol)/poly(acrylamide-co- diallyldimethylammonium chloride) as alkaline anion-exchange membrane for potential fuel cell applications. Synth. Met. 2013, 167, 43–50. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, J.; Jiang, G.; Liu, L.; Liu, Y. Cross-linked poly(vinyl alcohol)/poly (diallyldimethylammonium chloride) as anion-exchange membrane for fuel cell applications. J. Power Sources 2013, 240, 359–367. [Google Scholar] [CrossRef]
- Yuan, Y.; Shen, C.; Chen, J.; Ren, X. Synthesis and characterization of cross-linked quaternized chitosan/poly(diallyldimethylammonium chloride) blend anion-exchange membranes. Ionics (Kiel) 2018, 24, 1180. [Google Scholar] [CrossRef]
- Feketeföldi, B.; Cermenek, B.; Spirk, C.; Schenk, A.; Grimmer, C.; Bodner, M.; Koller, M.; Ribitsch, V.; Hacker, V. Chitosan-Based Anion Exchange Membranes for Direct Ethanol Fuel Cells. J. Membr. Sci. Technol. 2016, 06, 1–9. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, J.; Zhang, J. Anion conducting poly(vinyl alcohol)/poly(diallyldimethylammonium chloride) membranes with high durable alkaline stability for polymer electrolyte membrane fuel cells. J. Power Sources 2013, 237, 1–4. [Google Scholar] [CrossRef]
- Miraftab, M.; Saifullah, A.N.; Çay, A. Physical stabilisation of electrospun poly(vinyl alcohol) nanofibres: Comparative study on methanol and heat-based crosslinking. J. Mater. Sci. 2015, 50, 1943–1957. [Google Scholar] [CrossRef]
- Qiao, J.; Fu, J.; Liu, L.; Liu, Y.; Sheng, J. Highly stable hydroxyl anion conducting membranes poly(vinyl alcohol)/poly(acrylamide-co-diallyldimethylammonium chloride) (PVA/PAADDA) for alkaline fuel cells: Effect of cross-linking. Int. J. Hydrogen Energy 2012, 37, 4580–4585. [Google Scholar] [CrossRef]
- Yang, C.C.; Chiu, S.S.; Kuo, S.C.; Liou, T.H. Fabrication of anion-exchange composite membranes for alkaline direct methanol fuel cells. J. Power Sources 2012, 199, 37–45. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Ziv, N.; Dekel, D.R. A practical method for measuring the true hydroxide conductivity of anion exchange membranes. Electrochem. Commun. 2018, 88, 109–113. [Google Scholar] [CrossRef]
- Suzuki, S.; Muroyama, H.; Matsui, T.; Eguchi, K. Influence of CO2 dissolution into anion exchange membrane on fuel cell performance. Electrochim. Acta 2013, 88, 552–558. [Google Scholar] [CrossRef]
- Hari Gopi, K.; Bhat, S.D. Anion exchange membrane from polyvinyl alcohol functionalized with quaternary ammonium groups via alkyl spacers. Ionics (Kiel) 2018, 24, 1097–1109. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, Y.; Zhang, H.; Hou, L. Preparation and characterization of quaternized poly(vinyl alcohol)/chitosan/MoS2composite anion exchange membranes with high selectivity. Carbohydr. Polym. 2018, 180, 96–103. [Google Scholar] [CrossRef]
- García-Cruz, L.; Casado-Coterillo, C.; Irabien, Á.; Montiel, V.; Iniesta, J. High Performance of Alkaline Anion-Exchange Membranes Based on Chitosan/Poly (vinyl) Alcohol Doped with Graphene Oxide for the Electrooxidation of Primary Alcohols. C 2016, 2, 10. [Google Scholar] [CrossRef]
- Qiao, J.; Fu, J.; Liu, L.; Zhang, J.; Xie, J.; Li, G. Synthesis and properties of chemically cross-linked poly(vinyl alcohol)-poly(acrylamide-co-diallyldimethylammonium chloride) (PVA-PAADDA) for anion-exchange membranes. Solid State Ionics 2012, 214, 6–12. [Google Scholar] [CrossRef]








| NZ Content (%) | IEC (mmol·g−1) | WU (%) | SD (%) | TS (MPa) | Eb (%) | (mS·cm−1) |
|---|---|---|---|---|---|---|
| 0 | 0.54 | 102 | 43 | 6.73 | 138.32 | 27.3 |
| 1.0 | 0.52 | 93 | 43 | 7.21 | 134.34 | 28.3 |
| 1.5 | 0.52 | 89 | 42 | 10.87 | 245.27 | 31.6 |
| 2.0 | 0.57 | 84 | 40 | 10.13 | 207.64 | 26.5 |
| 2.5 | 0.52 | 73 | 29 | 13.96 | 228.87 | 28.9 |
| Materials | IEC (mmol·g−1) | Conductivity (mS·cm−1) | References |
|---|---|---|---|
| QPVA/HDT | 0.73 | 4.84 (30 °C) | [42] |
| QPVA/CS/MoS2 | 0.89 | 32 (25 °C) | [43] |
| QPVA/QCS | 1.75 | 16 (25 °C) | [34] |
| CS/PVA/GO | 0.38 | 0.19 (25 °C) | [44] |
| PVA/PDDA | 0.85 | 25 (25 °C) | [35] |
| PVA/PAADDA | 1.63 | 3 (25 °C) | [45] |
| QPVA/Q-SiO2 | 0.65 | 2.37 (25 °C) | [38] |
| PVA/PDDA/MWNT | 0.89 | 30.3 (30 °C) | [28] |
| PVA/PDDA/ZrO2 | 0.54 | 31.6 (RT) | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsudin, A.M.; Hacker, V. Preparation and Characterization of PVA/PDDA/Nano-Zirconia Composite Anion Exchange Membranes for Fuel Cells. Polymers 2019, 11, 1399. https://doi.org/10.3390/polym11091399
Samsudin AM, Hacker V. Preparation and Characterization of PVA/PDDA/Nano-Zirconia Composite Anion Exchange Membranes for Fuel Cells. Polymers. 2019; 11(9):1399. https://doi.org/10.3390/polym11091399
Chicago/Turabian StyleSamsudin, Asep Muhamad, and Viktor Hacker. 2019. "Preparation and Characterization of PVA/PDDA/Nano-Zirconia Composite Anion Exchange Membranes for Fuel Cells" Polymers 11, no. 9: 1399. https://doi.org/10.3390/polym11091399