Pore Structure and Properties of PEEK Hollow Fiber Membranes: Influence of the Phase Structure Evolution of PEEK/PEI Composite
Abstract
:1. Introduction
2. Experimental
2.1. Materials
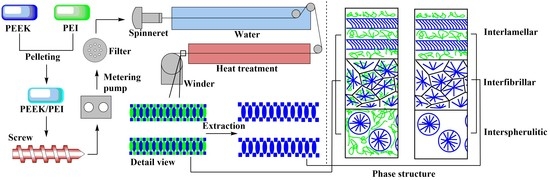
2.2. Melt Spinning of the Precursory PEEK/PEI Blend Hollow Fibers
2.3. Preparation of PEEK Hollow Fiber Membrane
2.4. Characterization of the Hollow Fiber Membrane
3. Results and Discussion
3.1. Phase Structure of PEEK and PEI
3.2. Effect of Phase Structure on Membrane Morphology
3.3. Formatting of Mathematical Components
3.4. Effect of Phase Structure on Porosity and Transport Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsuura, T. Progress in membrane science and technology for seawater desalination—A review. Desalination 2001, 134, 47–54. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Kaghazchi, T.; Kargari, A. Application of Membrane Separation Processes in Petrochemical Industry. Desalination 2009, 235, 199–244. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rusli, H.; Nath, A.; Sikder, J.; Bhattacharjee, C.; Curcio, S.; Drioli, E. Immobilized biocatalytic process development and potential application in membrane separation: A review. Crit. Rev. Biotechnol. 2016, 36, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.J.M. Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications. Int. J. Food Sci. Technol. 2010, 44, 1464–1466. [Google Scholar]
- Tan, X.; Liu, S.; Li, K. Preparation and characterization of inorganic hollow fiber membranes. J. Membr. Sci. 2001, 188, 87–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Run-Nan, G.U. The Application of Inorganic Ceramic Membrane in Environmental Water Treatment. J. Donghua Univ. Nat. Sci. 2005. [Google Scholar]
- Jiang, S.P. Functionalized mesoporous materials as new class high temperature proton exchange membranes for fuel cells. J. Mater. Chem. A 2014, 2, 7637–7655. [Google Scholar] [CrossRef]
- Liu, L.-F.; Gu, X.-L.; Xie, X.; Li, R.-H.; Yu, C.-Y.; Song, X.-X.; Gao, C.-J. Modification of PSf/SPSf Blended Porous Support for Improving the Reverse Osmosis Performance of Aromatic Polyamide Thin Film Composite Membranes. Polymers 2018, 10, 686. [Google Scholar] [CrossRef]
- Mozia, S.; Grylewicz, A.; Zgrzebnicki, M.; Darowna, D.; Czyzewski, A. Investigations on the Properties and Performance of Mixed-Matrix Polyethersulfone Membranes Modified with Halloysite Nanotubes. Polymers 2019, 11, 671. [Google Scholar] [CrossRef]
- Wang, S.; Ajji, A.; Guo, S.; Xiong, C. Preparation of Microporous Polypropylene/Titanium Dioxide Composite Membranes with Enhanced Electrolyte Uptake Capability via Melt Extruding and Stretching. Polymers 2017, 9, 110. [Google Scholar] [CrossRef]
- Ananth, A.; Arthanareeswaran, G.; Mok, Y.S. Effects of in situ and ex situ formations of silica nanoparticles on polyethersulfone membranes. Polym. Bull. 2014, 71, 2851–2861. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, Z.H.; Rudolph, V. Enhanced gas permeability by fabricating functionalized multi-walled carbon nanotubes and polyethersulfone nanocomposite membrane. Sep. Purif. Technol. 2011, 78, 76–82. [Google Scholar] [CrossRef]
- Patel, P.; Hull, T.R.; Mccabe, R.W.; Flath, D.; Grasmeder, J.; Percy, M. Mechanism of thermal decomposition of poly(ether ether ketone) (PEEK) from a review of decomposition studies. Polym. Degrad. Stab. 2010, 95, 709–718. [Google Scholar] [CrossRef] [Green Version]
- Shekar, R.I.; Kotresh, T.M.; Rao, P.M.D.; Kumar, K. Properties of high modulus PEEK yarns for aerospace applications. J. Appl. Polym. Sci. 2010, 112, 2497–2510. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Liu, X.; Dong, J.; Wang, J.; Yang, Z.; Cheng, H. Cross-linked fully aromatic sulfonated polyamide as a highly efficiency polymeric filler in SPEEK membrane for high methanol concentration direct methanol fuel cells. J. Mater. Sci. 2018, 53, 5501–5510. [Google Scholar] [CrossRef]
- Fu, X.; Matsuyama, H.; Teramoto, M.; Nagai, H. Preparation of polymer blend hollow fiber membrane via thermally induced phase separation. Sep. Purif. Technol. 2006, 52, 363–371. [Google Scholar] [CrossRef]
- Fu, X.; Matsuyama, H.; Teramoto, M.; Nagai, H. Preparation of hydrophilic poly(vinyl butyral) hollow fiber membrane via thermally induced phase separation. Sep. Purif. Technol. 2005, 45, 200–207. [Google Scholar] [CrossRef]
- Beck, H.N.; Lundgard, R.A.; Chau, C.C.; Wessling, R.A.; Mahoney, R.D. Film, Fiber, and Microporous Membrane Prepared from Poly(etheretherketone) Dissolved in High Boiling Point Polar Organic Solvents. U.S. Patent No. EP0407684(A1), 16 January 1991. [Google Scholar]
- Sonnenschein, M.F. Hollow Fiber Microfiltration Membranes from Poly(ether ether ketone) (PEEK). J. Appl. Polym. Sci. 2015, 72, 175–181. [Google Scholar] [CrossRef]
- Harris, J.E.; Robeson, L.M. Miscible Blends of poly(aryl ether ketone)s and polyetherimides. J. Appl. Polym. Sci. 2010, 35, 1877–1891. [Google Scholar] [CrossRef]
- Bicakci, S.; Cakmak, M. Development of structural hierarchy during uniaxial drawing of PEEK/PEI blends from amorphous precursors. Polymer 2002, 43, 149–157. [Google Scholar] [CrossRef]
- Hahn, B.; Wendorff, J.; Yoon, D.Y. Dielectric relaxation of the crystal-amorphous interphase in poly(vinylidene fluoride) and its blends with poly(methyl methacrylate). Macromolecules 1985, 18, 718–721. [Google Scholar] [CrossRef]
- Hahn, B.R.; Herrmannschonherr, O.; Wendorff, J.H. Evidence for a crystal-amorphous interphase in PVDF and PVDF/PMMA blends. Polymer 1987, 28, 201–208. [Google Scholar] [CrossRef]
- Mehta, R.H.; Kalika, D.S. Characteristics of poly(ether ether ketone) microporous membranes prepared via thermally induced phase separation (TIPS). J. Appl. Polym. Sci. 1997, 66, 2347–2355. [Google Scholar] [CrossRef]
- Bristow, J.F.; Kalika, D.S. Investigation of semicrystalline morphology in poly(ether ether ketone)/poly(ether imide) blends by dielectric relaxation spectroscopy. Polymer 1997, 38, 287–295. [Google Scholar] [CrossRef]
- Hudson, S.D.; Davis, D.D.; Lovinger, A.J. Semicrystalline morphology of poly(aryl ether ether ketone)/poly(ether imide) blends. Macromolecules 1992, 25, 1759–1765. [Google Scholar] [CrossRef]
- Mehta, R.H.; Madsen, D.A.; Kalika, D.S. Microporous membranes based on poly(ether ether ketone) via thermally-induced phase separation. J. Membr. Sci. 1995, 107, 93–106. [Google Scholar] [CrossRef]
- Ding, Y.; Bikson, B. Preparation and characterization of semi-crystalline poly(ether ether ketone) hollow fiber membranes. J. Membr. Sci. 2010, 357, 192–198. [Google Scholar] [CrossRef]
- Yang, M.C.; Liu, T.Y. The permeation performance of polyacrylonitrile/polyvinylidine fluoride blend membranes. J. Membr. Sci. 2003, 226, 119–130. [Google Scholar] [CrossRef]
- Rajabzadeh, S.; Maruyama, T. Preparation of PVDF/PMMA blend hollow fiber membrane via thermally induced phase separation (TIPS) method. Sep. Purif. Technol. 2009, 66, 76–83. [Google Scholar] [CrossRef]
- Liu, J.; Jungnickel, B.J. Composition distributions within poly(vinylidene fluoride) spherulites as grown in blends with poly(methyl methacrylate). J. Polym. Sci. Part B Polym. Phys. 2010, 44, 338–346. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Pérez-RamíRez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Labani, M.M.; Rezaee, R.; Saeedi, A.; Hinai, A.A. Evaluation of pore size spectrum of gas shale reservoirs using low pressure nitrogen adsorption, gas expansion and mercury porosimetry: A case study from the Perth and Canning Basins, Western Australia. J. Pet. Sci. Eng. 2013, 112, 7–16. [Google Scholar] [CrossRef]










| Annealing Temperature (°C) | Thickness (µm) | Pore Diameter (nm) | Surface Area (m2/g) | Porosity 1 (%) | Water Flux (Lh−1 m−2 bar−1) |
|---|---|---|---|---|---|
| 240 | 69.63 | 10.59 | 111.9 | 55.98 | 1.91 × 10−2 |
| 250 | 15.94 | 99.50 | 56.88 | 1.30 × 10−1 | |
| 260 | 24.43 | 96.13 | 58.67 | 1.47 × 10−1 | |
| 270 | 24.43 | 83.61 | 59.56 | 1.65 × 10−1 | |
| 280 | 37.85 | 83.69 | 59.11 | --- |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Chen, Y.; Huang, T.; He, Z.; Xu, J.; Liu, P. Pore Structure and Properties of PEEK Hollow Fiber Membranes: Influence of the Phase Structure Evolution of PEEK/PEI Composite. Polymers 2019, 11, 1398. https://doi.org/10.3390/polym11091398
Chen G, Chen Y, Huang T, He Z, Xu J, Liu P. Pore Structure and Properties of PEEK Hollow Fiber Membranes: Influence of the Phase Structure Evolution of PEEK/PEI Composite. Polymers. 2019; 11(9):1398. https://doi.org/10.3390/polym11091398
Chicago/Turabian StyleChen, Gong, Yuan Chen, Tingjian Huang, Zhongchen He, Jianjun Xu, and Pengqing Liu. 2019. "Pore Structure and Properties of PEEK Hollow Fiber Membranes: Influence of the Phase Structure Evolution of PEEK/PEI Composite" Polymers 11, no. 9: 1398. https://doi.org/10.3390/polym11091398