Synthesis and Properties of In-Situ Bulk High Impact Polystyrene Toughened by High cis-1,4 Polybutadiene
Abstract
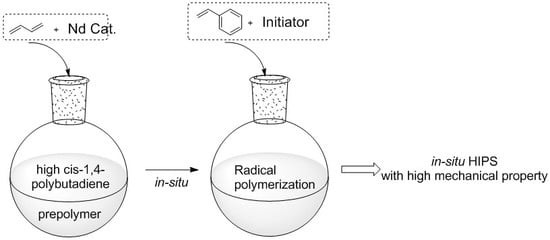
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Catalyst
2.3. Preparation of Butadiene Prepolymer Solution
2.4. Synthesis of HIPS Resins
2.5. Measurements and Characterization
2.6. Determination of Grafting Parameters
2.7. Notched IZOD Impact Testing
2.8. Tensile Testing
2.9. Transmission Electron Microscopy (TEM)
3. Results and Discussion
3.1. Polymerization of Butadiene
3.2. Effect of Bd Prepolymer Content on the Morphologies and Properties of HIPS
3.3. Effect of Initiator Concentration on the Morphologies and Properties of HIPS
3.4. Effect of Initiator Type on the Morphologies and Properties of HIPS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chang, S.Q.; Xie, T.X.; Yang, G.S. Morphology and mechanical properties of high-impact polystyrene/elastomer/magnesium hydroxide composites. J. Appl. Polym. Sci. 2006, 102, 5184–5190. [Google Scholar] [CrossRef]
- Rovere, J.; Correa, C.A.; Grassi, V.G.; Pizzaol, M.F.D. Role of the rubber particle and polybutadiene cis content on the toughness of high impact polystyrene. J. Mater. Sci. 2008, 43, 952–959. [Google Scholar] [CrossRef]
- León, R.D.; Morales, G.; Acuña, P.; Olivo, J.; Ramos-DeValle, L.F. Mechanical behavior of high impact polystyrene based on SBR copolymers: Part I. Polym. Eng. Sci. 2005, 45, 1288–1296. [Google Scholar] [CrossRef]
- Windisch, H.; Obrecht, W.; Michels, G.; Steinhauser, N. Method for Polymerizing Conjugated Diolefins (Dienes) with Catalysts Based on Cobalt Compounds in the Presence of Vinylaromatic Solvents. U.S. Patent 6,310,151 B1, 30 October 2001. [Google Scholar]
- Meria, G.R.; Luciani, C.V.; Estenoz, D.A. Continuous Bulk Process for the Production of High-Impact Polystyrene: Recent Developments in Modeling and Control. Macromol. React. Eng. 2007, 1, 25–39. [Google Scholar] [CrossRef]
- Alfarraj, A.; Nauman, E.B. Super HIPS: Improved high impact polystyrene with two sources of rubber particles. Polymer 2004, 45, 8435–8442. [Google Scholar] [CrossRef]
- Estenoz, D.A.; Valdez, E.; Oliva, H.; Meria, G.R. Bulk polymerization of styrene in presence of polybutadiene: Calculation of molecular macrostructure. J. Appl. Polym. Sci. 1996, 59, 861–885. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-based Ziegler/Natta catalysts and their application in diene polymerization. Adv. Polym. Sci. 2006, 204, 1–154. [Google Scholar]
- Hu, Y.; Jia, Z.; Li, Y.; Chang, L.; Wang, Y. Synthesis and impact properties of in situ bulk made ABS resins toughened by high cis-1, 4 polybutadiene. Mater. Sci. Eng. A 2011, 528, 6667–6672. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Y.; Wang, Y.; Yang, L.; Liu, Y.; Li, Y.; Li, Z.; Zhao, Z. Morphological, mechanical properties, and fracture behavior of bulk-made ABS resins toughened by high-cis polybutadiene rubber. Polym. Eng. Sci. 2010, 50, 961–969. [Google Scholar] [CrossRef]
- Luciani, C.V.; Estenoz, D.A.; Meira, G.R.; García, N.L.; Oliva, H.M. Bulk High-Impact Polystyrene Process, 1: Partitions of tert-Butyl Peroctoate and Styrene in Blends Containing Polystyrene and a Rubber. Macromol. Theory Simul. 2007, 16, 703–710. [Google Scholar] [CrossRef]
- Soto, G.; Nava, E.; Rosas, M.; Fuenmayor, M.; González, I.M.; Meira, G.R.; Oliva, H.M. Bulk polymerization of styrene in the presence of polybutadiene: Effect of initiator type and prepolymerization conditions on particle morphology. J. Appl. Polym. Sci. 2004, 92, 1397–1412. [Google Scholar] [CrossRef]
- Choi, J.H.; Ahn, K.H.; Kim, S.Y. Effects of the degree of graft on the tensile and dynamic behavior of high impact polystyrene. Polymer 2000, 41, 5229–5236. [Google Scholar] [CrossRef]
- Sharma, R.; Socrate, S. Micromechanics of uniaxial tensile deformation and failure in high impact polystyrene (HIPS). Polymer 2009, 50, 3386–3395. [Google Scholar] [CrossRef]
- Friebe, L.; Windisch, H.; Nuyken, O.; Obrecht, W. Polymerization of 1,3-Butadiene Initiated by Neodymium Versatate/Triisobutylaluminum/Ethylaluminum Sesquichloride: Impact of the Alkylaluminum Cocatalyst Component. J. Macromol. Sci. Pure Appl. Chem. 2004, A41, 245–256. [Google Scholar] [CrossRef]
- Yu, B.S. The infrared analysis of synthetic rubber structure and composition. Chin. J. Anal. Chem. 1974, 4, 311–322. (In Chinese) [Google Scholar]
- Bucknall, C.B.; Davies, P.; Partridge, I.K. Rubber toughening of plastics. J. Mater. Sci. 1987, 22, 1341–1346. [Google Scholar] [CrossRef]
- Cigna, G.; Lomellini, P.; Merlotti, M. Impact thermoplastics: Combined role of rubbery phase volume and particle size on toughening efficiency. J. Appl. Polym. Sci. 1989, 37, 1527–1540. [Google Scholar] [CrossRef]
- Li, D.; Peng, J.; Zhai, M.; Qiao, J.; Zhang, X.; Wei, G. Novel methods for synthesis of high-impact polystyrene with bimodal distribution of rubber particle size. J. Appl. Polym. Sci. 2008, 109, 2071–2075. [Google Scholar] [CrossRef]
- Turley, S.G.; Keskkula, H. Effect of rubber-phase volume fraction in impact polystyrene on mechanical behaviour H. Polymer 1980, 21, 466–468. [Google Scholar] [CrossRef]
- Xu, X.; Wang, R.; Tan, Z.; Yang, H.; Zhang, M.; Zhang, H. Effects of polybutadiene-g-SAN impact modifiers on the morphology and mechanical behaviors of ABS blends. Eur. Polym. J. 2005, 41, 1919–1926. [Google Scholar] [CrossRef]
- Leal, G.P.; Asua, J.M. Evolution of the morphology of HIPS particles. Polymer 2009, 50, 68–76. [Google Scholar] [CrossRef]
- Sheng, W.; Wu, J.; Shan, G.; Huang, Z.M.; Weng, Z.X. Free-radical bulk polymerization of styrene with a new trifunctional cyclic peroxide initiator. J. Appl. Polym. Sci. 2004, 94, 1035–1042. [Google Scholar] [CrossRef]
- Fityani-Trimm, S.; Dhib, R.; Penlidis, A. Free radical polymerization of styrene with a new tetrafunctional peroxide initiator. Macromol. Chem. Phys. 2003, 204, 436–442. [Google Scholar] [CrossRef]





| Conversion (%) | St Content b (mol %) | Microstructure c (%) | Mw × 10−4 d | Mw/Mnd | |||
|---|---|---|---|---|---|---|---|
| Bd | St | cis-1,4 | trans-1,4- | 1,2- | |||
| 100 | 0.5 | 1.01 | 96.6 | 2.6 | 0.8 | 17.7 | 3.05 |
| PB (wt %) | RPVF (%) | GD (%) | PS | Mechanical Properties | ||
|---|---|---|---|---|---|---|
| Mw × 10−4 b | Mw/Mnb | Impact Strength (J/m) | Tensile Strength at Break (MPa) | |||
| 5.0 | 10.0 | 78.1 | 21.8 | 1.76 | 48.5 | 16.5 |
| 7.5 | 14.3 | 84.7 | 20.8 | 1.86 | 84.8 | 16.8 |
| 10.0 | 19.6 | 85.0 | 22.9 | 1.87 | 86.0 | 16.7 |
| 15.0 | 35.8 | 117.9 | 17.4 | 2.24 | 166.2 | 12.5 |
| TETMTPA (wt %) | RPVF (%) | GD (%) | PS | Mechanical Properties | ||
|---|---|---|---|---|---|---|
| Mw × 10−4 b | Mw/Mnb | Impact Strength (J/m) | Tensile Strengthat Break (MPa) | |||
| 0.01 | 17.2 | 50.4 | 22.6 | 1.85 | 120.6 | 16.1 |
| 0.02 | 18.3 | 68.2 | 22.6 | 1.89 | 107.4 | 16.0 |
| 0.03 | 19.6 | 85.0 | 22.9 | 1.87 | 86.0 | 16.7 |
| 0.05 | 23.8 | 131.4 | 20.7 | 1.80 | 72.4 | 13.2 |
| Initiator | RPVF (%) | GD (%) | PS | Mechanical Properties | |||
|---|---|---|---|---|---|---|---|
| Type | Content (wt %) | Mw × 10−4 b | Mw/Mnb | Impact Strength (J/m) | Tensile Strengthat Break (MPa) | ||
| BPO | 0.090 | 22.1 | 92.1 | 22.2 | 1.72 | 19.2 | 18.3 |
| DP275B | 0.045 | 28.2 | 167.1 | 34.3 | 1.70 | 45.8 | 16.0 |
| TETMTPA | 0.030 | 14.3 | 84.7 | 20.8 | 1.86 | 84.8 | 16.8 |
| TBPP | 0.023 | 36.8 | 195.6 | 27.3 | 1.69 | 13.5 | 20.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Chang, L.; Hu, Y.; Wu, G.; Liu, H. Synthesis and Properties of In-Situ Bulk High Impact Polystyrene Toughened by High cis-1,4 Polybutadiene. Polymers 2019, 11, 791. https://doi.org/10.3390/polym11050791
Wang F, Chang L, Hu Y, Wu G, Liu H. Synthesis and Properties of In-Situ Bulk High Impact Polystyrene Toughened by High cis-1,4 Polybutadiene. Polymers. 2019; 11(5):791. https://doi.org/10.3390/polym11050791
Chicago/Turabian StyleWang, Feng, Li Chang, Yanming Hu, Guangfeng Wu, and Heng Liu. 2019. "Synthesis and Properties of In-Situ Bulk High Impact Polystyrene Toughened by High cis-1,4 Polybutadiene" Polymers 11, no. 5: 791. https://doi.org/10.3390/polym11050791