Ablation Behavior of Silicone Rubber-Benzoxazine-Based Composites for Ultra-High Temperature Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparation
2.3. Characterization and Ablation Experiment
2.3.1. Ablation Experiment
2.3.2. Sample Characterization
3. Results and Discussion
3.1. TGA
3.2. Ablation Properties of the Composites
3.3. Burnt Structure and Composition Analysis of the Composites
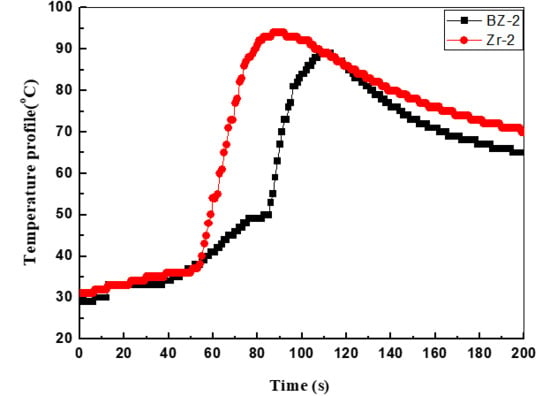
3.4. The Back Surface Temperatures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balaji, R.; Sasikumar, M. A study on the effect of cenosphere on thermal and ablative behavior of cenosphere loaded ceramic/phenolic composites. Polymer 2014, 55, 6634–6639. [Google Scholar]
- Natali, M.; Rallini, M.; Puglia, D.; Kenny, J.; Torre, L. EPDM based heat shielding materials for Solid Rocket Motors: A comparative study of different fibrous reinforcements. Polym. Degrad. Stab. 2013, 98, 2131–2139. [Google Scholar] [CrossRef]
- Bahramian, A.R.; Kokabi, M. Ablation mechanism of polymer layered silicate nanocomposite heat shield. J. Hazard. Mater. 2009, 166, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, C.; Su, J.; Li, Y.; Yin, Z. Ablation and mechanical investigation of carbon/rubber woven laminates for ultrahigh temperature applications. Int. J. Mater. Res. 2018, 109, 673–676. [Google Scholar] [CrossRef]
- Guo, M.; Li, J.; Xi, K.; Liu, Y.; Ji, J. Effect of multi-walled carbon nanotubes on thermal stability and ablation properties of EPDM insulation materials for solid rocket motors. Acta Astronaut. 2019, 159, 508–516. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Jiang, B.; Guo, Y. Silicone rubber ablative composites improved with zirconium carbide or zirconia. Compos. Part A 2013, 44, 70–77. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.; Li, Y.; Yin, Z.; Zhi, Y.; Gao, J. Ablative properties and mechanisms of hot vulcanised silicone rubber (HVSR) composites containing different fillers. Plast. Rubber Compos. 2016, 45, 430–435. [Google Scholar] [CrossRef]
- Yao, W.J.; Cao, B.Y. Triggering wave-domain heat conduction in graphene. Phys. Lett. A 2016, 380, 2105–2110. [Google Scholar] [CrossRef]
- Liu, T.; Zeng, X.; Lai, X.; Li, H.; Wu, T. Improvement of platinum nanoparticles-immobilized α-zirconium phosphate sheets on tracking and erosion resistance of silicone rubber. Compos. Part B 2019, 176, 107203. [Google Scholar] [CrossRef]
- Song, Y.; Yang, R.; Du, M.; Shi, X.; Zheng, Q. Rigid nanoparticles promote the softening of rubber phase in filled vulcanizates. Polymer 2019, 177, 131–138. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, W.; Liu, Y.; Song, H.; Huang, C. High-power laser resistance of filled sandwich panel with truss cores: Ablation mechanisms and numerical simulation. Compos. Struct. 2018, 203, 574–584. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, E.J.; Shim, J.H.; Yoon, J.S. Thermal stability and ablation properties of silicone rubber composites. J. Appl. Polym. Sci. 2008, 110, 1263–1270. [Google Scholar] [CrossRef]
- Barile, C.; Casavola, C.; De Cillis, F. Mechanical comparison of new composite materials for aerospace applications. Compos. Part B 2019, 162, 122–128. [Google Scholar] [CrossRef]
- Chen, L.; Ren, D.; Chen, S.; Pan, H.; Xu, M.; Liu, X. Copolymerization of phthalonitrile-based resin containing benzoxazine and cyanate ester: Curing behaviors, fiber-reinforced composite laminates and improved properties. Express Polym. Lett. 2019, 13, 456–468. [Google Scholar] [CrossRef]
- Comer, A.J.; Ray, D.; Clancy, G.; Obande, W.O.; Rosca, I.; McGrail, P.T.; Stanley, W.F. Hydrothermal in-plane-shear strength of carbon fibre/benzoxazine laminates manufactured out-of-autoclave by liquid-resin-infusion. Compos. Struct. 2019, 213, 261–270. [Google Scholar] [CrossRef]
- Dogan, Y.E.; Satilmis, B.; Uyar, T. Synthesis and characterization of bio-based benzoxazines derived from thymol. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Liu, Y.; Su, J.; Yin, Z.; Li, Y.; Zhi, Y.; Gao, J. Ablation and mechanical investigation of heat vulcanizing silicone rubber (HVSR) composite containing carbon fibers. J. Polym. Eng. 2017, 37, 521–528. [Google Scholar] [CrossRef]






| Sample | PDMS (phr) | PMPS (phr) | SiO2 (phr) | BZs (phr) | CF (phr) | ZrO2 (phr) |
|---|---|---|---|---|---|---|
| BZ-0 | 50 | 50 | 30 | 0 | 10 | 0 |
| BZ-1 | 50 | 50 | 30 | 10 | 10 | 0 |
| BZ-2 | 50 | 50 | 30 | 20 | 10 | 0 |
| BZ-3 | 50 | 50 | 30 | 30 | 10 | 0 |
| BZ-4 | 50 | 50 | 30 | 40 | 10 | 0 |
| Zr-1 | 50 | 50 | 30 | 20 | 10 | 5 |
| Zr-2 | 50 | 50 | 30 | 20 | 10 | 10 |
| Zr-3 | 50 | 50 | 30 | 20 | 10 | 15 |
| Zr-4 | 50 | 50 | 30 | 20 | 10 | 20 |
| Specimens | O (%) | Si (%) | Zr (%) | C (%) |
|---|---|---|---|---|
| BZ-2 | 54.11 | 43.66 | - | 2.23 |
| Zr-2 | 42.91 | 46.25 | 6.87 | 3.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Li, Z.; Li, J.; Liu, Y. Ablation Behavior of Silicone Rubber-Benzoxazine-Based Composites for Ultra-High Temperature Applications. Polymers 2019, 11, 1844. https://doi.org/10.3390/polym11111844
Gao J, Li Z, Li J, Liu Y. Ablation Behavior of Silicone Rubber-Benzoxazine-Based Composites for Ultra-High Temperature Applications. Polymers. 2019; 11(11):1844. https://doi.org/10.3390/polym11111844
Chicago/Turabian StyleGao, Jinglong, Zhixuan Li, Jiayi Li, and Yanhui Liu. 2019. "Ablation Behavior of Silicone Rubber-Benzoxazine-Based Composites for Ultra-High Temperature Applications" Polymers 11, no. 11: 1844. https://doi.org/10.3390/polym11111844