Facile Functionalization of Poly(Dimethylsiloxane) Elastomer by Varying Content of Hydridosilyl Groups in a Crosslinker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(Dimethylsiloxane-co-methylvinylsiloxane) (VPDMS)
2.3. Synthesis of Poly(Dimethylsiloxane-co-methylsiloxane) (HPDMS)
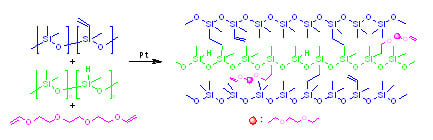
2.4. Identification of Reaction of TEGDE with HPDMS
2.5. Preparation of PDMS Dope and Film Fabrication
2.6. Measurements
3. Results and Discussion
3.1. Synthesis and Characterization of VPDMS and HPDMS
3.2. Curing Condition of Crosslinking Between VPDMS and HPDMS
3.3. Fabtication of PDMS Elastomer Films Containing TEGDE
3.4. Electrical, Optical, and Mechanical Properties of the PDMS Elastomer Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Duduta, M.; Hajiesmaili, E.; Zhao, H.; Wood, R.J.; Clarke, D.R. Realizing the potential of dielectric elastomer artificial muscles. Proc. Natl. Acad. Sci. USA 2019, 116, 2476–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Li, G.; Liang, Y.; Cheng, T.; Dai, J.; Yang, X.; Liu, B.; Zeng, Z.; Huang, Z.; Luo, Y.; et al. Fast-moving soft electronic fish. Sci. Adv. 2017, 3, e1602045. [Google Scholar] [CrossRef] [PubMed]
- Brochu, P.; Pei, Q. Advances in dielectric elastomers for actuators and artificial muscles. Macromol. Rapid Commun. 2010, 31, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.; Yun, S.; Nam, S.; Park, S.K.; Park, S.; Park, B.J.; Lim, J.M.; Kyung, K.-U. Electro-active polymer based soft tactile interface for wearable devices. IEEE Trans. Haptics 2018, 11, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Yun, S.; Yoon, J.W.; Park, S.; Park, S.K.; Mun, S.; Park, B.; Kyung, K.-U. A robust soft lens for tunable camera application using dielectric elastomer actuators. Soft Robot. 2018, 5, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Speier, J.L.; Webster, J.A.; Barnes, G.H. The addition of silicone hydrides to olefinic double bonds. Part II. The use of group VIII metal catalysts. J. Am. Chem. Soc. 1957, 79, 974–979. [Google Scholar] [CrossRef]
- Zheng, P.; McCarthy, T.J. Rediscovering silicones: Molecular smooth, low surface energy, unfilled, UV/Vis-transparent, extremely crosslinked, thermally stable, hard, elastic PDMS. Langmuir 2010, 26, 18585–18590. [Google Scholar] [CrossRef]
- Mark, J.E. Some interesting things about polysiloxanes. Acc. Chem. Res. 2004, 37, 946–953. [Google Scholar] [CrossRef]
- Rolland, J.P.; Van Dam, R.M.; Schorzman, D.A.; Quake, S.R.; DeSimone, J.M. Solvent-resistant photocurable “liquid Teflon” for microfluidic device fabrication. J. Am. Chem. Soc. 2004, 126, 2322–2323. [Google Scholar] [CrossRef]
- Tanaka, Y. A peristaltic pump integrated on a 100% glass microchip using computer controlled piezoelectric actuators. Micromachines 2014, 5, 289–299. [Google Scholar] [CrossRef]
- Yildirim, E.; Sahir Arikan, M.A.; Külah, H. A normally closed electrostatic parylene microvalve for micro total analysis systems. Sens. Actuator A-Phys. 2012, 181, 81–86. [Google Scholar] [CrossRef]
- Caspari, P.; Dünki, S.J.; Nüesch, F.A.; Opris, D.M. Dielectric elastomer actuators with increased dielectric permittivity and low leakage current capable of suppressing electromechanical instability. J. Mater. Chem. C 2018, 6, 2043–2053. [Google Scholar] [CrossRef]
- Madsen, F.B.; Yu, L.; Daugaard, A.E.; Hvilsted, S.; Skov, A.L. A new soft dielectric silicone elastomer matrix with high mechanical integrity and low losses. RSC Adv. 2015, 5, 10254–10259. [Google Scholar] [CrossRef] [Green Version]
- Dascalu, M.; Dünki, S.J.; Quinssat, J.-E.; Ko, Y.S.; Opris, D.M. Synthesis of silicone elastomers containing trifluoropropyl groups and their use in dielectric elastomer transducers. RSC Adv. 2015, 5, 104516–104523. [Google Scholar] [CrossRef]
- Racles, C.; Bele, A.; Dascalu, M.; Musteata, V.E.; Varganici, C.D.; Ionita, D.; Vlad, S.; Cazacu, M.; Dünki, S.J.; Opris, D.M. Polar–nonpolar interconnected elastic networks with increased permittivity and high breakdown fields for dielectric elastomer transducers. RSC Adv. 2015, 5, 58428–58438. [Google Scholar] [CrossRef]
- Madsen, F.B.; Daugaard, A.E.; Hvilsted, S.; Skov, A.L. The current state of silicone-based dielectric elastomer transducers. Macromol. Rapid Commun. 2016, 37, 378–413. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Yang, D.; Yu, Y.; Yao, L.; Tian, M. Mechanical, dielectric, and actuated strain of silicone elastomer filled with various types of TiO2. Soft Mater. 2013, 11, 363–370. [Google Scholar] [CrossRef]
- Stiubianu, G.; Bele, A.; Cazacu, M.; Racles, C.; Vlad, S.; Ignat, M. Dielectric silicone elastomers with mixed ceramic nanoparticles. Mater. Res. Bull. 2015, 71, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Bele, A.; Cazacu, M.; Stiubianu, G.; Vlad, S.; Ignat, M. Polydimethylsiloxane–barium titanate composites: Preparation and evaluation of the morphology, moisture, thermal, mechanical and dielectric behavior. Compos. Pt. B-Eng. 2015, 68, 237–245. [Google Scholar] [CrossRef]
- Lee, Y.J.; Caspari, P.; Opris, D.M.; Nüesch, F.A.; Ham, S.; Kim, J.-H.; Kim, S.-R.; Ju, B.-K.; Choi, W.K. Electrical energy generated by silicone elastomers filled with nanospring-carbon-nanotubes. J. Mater. Chem. C 2019, 7, 3535–3542. [Google Scholar] [CrossRef]
- Guan, S.; Song, S.; Li, H.; Mo, G.; Zhao, S.; Guo, L. Development of carboxyl-functionalized multi-walled nanotube/polydimethylsiloxane novel polymeric nanodielectric material. Mater. Lett. 2018, 216, 281–286. [Google Scholar] [CrossRef]
- Quinssat, J.E.Q.; Alexandru, M.; Nüesch, F.A.; Hofmann, H.; Borgschulte, A.; Opris, D.M. Highly stretchable dielectric elastomer composites containing high volume fractions of silver nanoparticles. J. Mater. Chem. A 2015, 3, 14675–14685. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Hu, P.; Zha, J.-W.; You, F.; Li, S.-T.; Dang, Z.-M. Highly improved electro-actuation of dielectric elastomers by molecular grafting of azobenzenes to silicon rubber. J. Mater. Chem. C 2015, 3, 4883–4889. [Google Scholar] [CrossRef]
- Moon, H.K.; Kang, S.; Yoon, H.J. Aziridine-functionalized polydimethylsiloxanes for tailorable polymeric scaffolds: Aziridine as a clickable moiety for structural modification of materials. Polym. Chem. 2017, 8, 2287–2291. [Google Scholar] [CrossRef]
- Pelrine, R.; Kornbluh, R.; Pei, Q.; Joseph, J. High-speed electrically actuated elastomers with strain greater than 100%. Science 2000, 287, 836–839. [Google Scholar] [CrossRef]
- Dorfmann, L.; Ogden, R.W. Nonlinear electroelasticity: Material properties, continuum theory and applications. Proc. R. Soc. A-Math. Phys. Eng. Sci. 2017, 473, 20170311. [Google Scholar] [CrossRef]
- Mehnert, M.; Hossain, M.; Steinmann, P. Experimental and numerical investigations of the electro-viscoelastic behavior of VHB 4905TM. Eur. J. Mech. A-Solids 2019, 77, 103797. [Google Scholar] [CrossRef]
- Hossain, M.; Steinmann, P. Modelling electro-active polymers with a dispersion-type anisotropy. Smart Mater. Struct. 2018, 27, 025010. [Google Scholar] [CrossRef]
- Takamura, N.; Gunji, T.; Hatano, H.; Abe, Y. Preparation and properties of polysilsesquioxanes: Polysilsesquioxanes and flexible thin films by acid-catalyzed controlled hydrolytic polycondensation of methyl and vinyltrimethoxysilane. J. Polym. Sci. Polym. Chem. 1999, 37, 1017–1026. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities-properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Touzi, H.; Chevalier, Y.; Kalfat, R.; Jaffrezic-Renault, N. New elastomeric polymethylsiloxane membranes bearing cationic exchanging sites for anionic dyestuffs sensors. Eur. Polym. J. 2014, 56, 140–158. [Google Scholar] [CrossRef]
- Li, J.P.; Cassagnau, P.; Da Cruz-Boisson, F.; Mélis, F.; Alcouffe, P.; Bounor-Legaré, V. Efficient hydrosilylation reaction in polymer blending: An original approach to structure PA12/PDMS blends at multiscales. Polymer 2017, 112, 10–25. [Google Scholar] [CrossRef]
- Risangud, N.; Li, Z.; Anastasaki, A.; Wilson, P.; Kempe, K.; Haddleton, D. Hydrosilylation as an efficient tool for polymer synthesis and modification with methacrylates. RSC Adv. 2015, 5, 5879–5885. [Google Scholar] [CrossRef]
- Dirany, M.; Dies, L.; Restagno, F.; Léger, L.; Poulard, C.; Miquelard-Garnier, G. Chemical modification of PDMS surface without impacting the viscoelasticity: Model systems for a better understanding of elastomer/elastomer adhesion and friction. Colloid Surf. A-Physicochem. Eng. Asp. 2015, 468, 174–183. [Google Scholar] [CrossRef]
- Yan, L.; Li, J.; Liu, N.; Hao, X.; Li, C.; Hou, W.; Li, D.X. Thermostable gold nanoparticle-doped silicone elastomer for optical materials. Colloid Surf. A-Physicochem. Eng. Asp. 2017, 518, 151–157. [Google Scholar] [CrossRef]
- Park, S.K.; Farris, R.J. Dry-jet wet spinning of aromatic polyamic acid fiber using chemical imidization. Polymer 2001, 42, 10087–10093. [Google Scholar] [CrossRef]
- Park, S.K.; Kwark, Y.-J.; Nam, S.; Park, S.; Park, B.; Yun, S.; Moon, J.; Lee, J.-I.; Yu, B.; Kyung, K.-U. Wrinkle structures formed by formulating UV-crosslinkable liquid prepolymers. Polymer 2016, 99, 447–452. [Google Scholar] [CrossRef]
- SylgardTM 184 Silicone Elastomer Kit Technical Data Sheet. Available online: https://www.dow.com/en-us/document-viewer.html?ramdomVar=6429644214938805507&docPath=/content/dam/dcc/documents/en-us/productdatasheet/11/11-31/11-3184-sylgard-184-elastomer.pdf (accessed on 4 November 2019).



| Name of Copolymer | Mn (g/mol) 1 | Mw (g/mol) 1 | l or n (Mole Ratio) | m or o (Mole Ratio) |
|---|---|---|---|---|
| VPDMS | 100,741 | 165,425 | 0.990 | 0.010 |
| HPDMS10 | 16,814 | 97,048 | 0.925 | 0.075 |
| HPDMS20 | 16,414 | 38,480 | 0.807 | 0.193 |
| Name of Polymer Dope | VPDMS (g) | TEGDE (g) | HPDMS10 (g) | HPDMS20 (g) | Initial Modulus 1 (kPa) (s.d.) 2 | Maximum Stress 1 (kPa) (s.d.) | Maximum Strain 1 (%) (s.d.) |
|---|---|---|---|---|---|---|---|
| VH10 | 1.3769 | - | 0.1589 (1.14) 3 | - | 762 (54) | 926 (234) | 156 (30) |
| VH10T | 1.4542 | 0.1685 | 0.1697 (1.12) 3 | - | 154 (17) | 299 (25) | 265 (18) |
| VH20T | 1.3554 | 0.1547 | - | 0.1509 (0.448) 3 | 484 (36) | 1,438 (141) | 277 (10) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.K.; Park, B.J.; Choi, M.J.; Kim, D.W.; Yoon, J.W.; Shin, E.J.; Yun, S.; Park, S. Facile Functionalization of Poly(Dimethylsiloxane) Elastomer by Varying Content of Hydridosilyl Groups in a Crosslinker. Polymers 2019, 11, 1842. https://doi.org/10.3390/polym11111842
Park SK, Park BJ, Choi MJ, Kim DW, Yoon JW, Shin EJ, Yun S, Park S. Facile Functionalization of Poly(Dimethylsiloxane) Elastomer by Varying Content of Hydridosilyl Groups in a Crosslinker. Polymers. 2019; 11(11):1842. https://doi.org/10.3390/polym11111842
Chicago/Turabian StylePark, Seung Koo, Bong Je Park, Mee Jeong Choi, Dong Wook Kim, Jae Woong Yoon, Eun Jin Shin, Sungryul Yun, and Suntak Park. 2019. "Facile Functionalization of Poly(Dimethylsiloxane) Elastomer by Varying Content of Hydridosilyl Groups in a Crosslinker" Polymers 11, no. 11: 1842. https://doi.org/10.3390/polym11111842