1. Introduction
Generally, the assessment of fire risks for the new materials involves various approaches for collating different fire-related parameters [
1,
2]. They include, but not limited to, the radiant heat flux generated, the amounts of smoke and toxic gaseous components produced (e.g., carbon monoxide CO and carbon dioxide CO
2), the fire spread rate and the propensity of construction elements to result in structural failures. The associated material’s properties of interest are times (or temperatures) to ignition; heat release rates (HRRs); heat release capacities (HRCs); char residues; and kinetic parameters pertaining to the thermal degradation [
3]. In order to measure the experimental quantities of interest, a variety of small-, medium-, and large-scale tests are usually performed. These include: A corner room test ISO 9705 (large-scale) [
4]), cone calorimetry (medium-scale) [
5]), and small-scale tests such as thermo-gravimetric analysis (TGA) [
6] and pyrolysis combustion flow calorimetry (PCFC) [
7].
There have been several attempts to find correlations between the empirical data obtained through small- and medium-scale tests [
8] with a view to predicting an overall reaction of materials to real-life fires. For instance, Östman and Nussbaum found an empirical correlation between the times to flashover in a full-scale room fire test for surface lining materials from the measurements of the rate of heat release in a cone calorimeter [
9]. Furthermore, cone calorimetry results were employed by Wickström and Göransson to predict the heat release rate of surface materials in a large-scale fire test [
10]. Dowling and Feske estimated the fire spread and smoke evolution of flame-retarded rigid polyurethane (FR-PUR) from the relevant cone data [
11].
However, not all of these attempts were fully successful. For example, Weil et al. (1992) attempted, with a limited success, to correlate the cone calorimetry measurements and the limiting oxygen indices (LOIs) [
12]. Similarly, Hirschler had found only partial correlations among various data obtained through studies of the HRRs for twenty-one types of electrical cables, using several small-scale fire tests (e.g., various small burner-type and cone calorimeter tests), three European medium-scale cable tray tests (DIN 4102, Brandschacht; BS 476/Part 12 Ignition source E; IEC 1034), and two large-scale vertical cable tray tests (ASTM D5424#5537 (CSA FT4) and IEC 332-3) [
13].
The correlation studies between the results from TGA (both in air and in nitrogen) of nine different ‘flame-resistant’ and one ‘non-flame resistant’ rubber conveyor belts, LOI measurements, hot plate ignition, and drum friction tests (i.e., other flammability tests) were also reported in the literature [
14]. Generally, the relevant empirical parameters such as activation energy and pre-exponential factor are very useful in validating some existing mathematical/computational models pertaining to the ignition characteristics and fire behaviours of the materials. For instance, these kinetic parameters can be used for combustion and pyrolysis sub-models of a computational model, simulating the fire spread on a defined woodland [
3].
Through the current research, we undertook various experimental testing to gain insights into the ignition behaviour and combustion characteristics of two materials that are commonly used in the construction sector: Cross-laminated timber (CLT) and the inner core of aluminium composite panels (ACPs). In recent years, the chosen materials were severely implicated in the initiation, or the spread of fires, in enclosures and exteriors of the buildings [
1,
2]. For example, ACPs for external façades caught the attention of the scientific community following the Grenfell Tower fire in London [
1]. It is believed that a flammable polyethylene-based core of ACPs has contributed to a rapid fire spread outside the tower block. Other examples of fires with similar materials involved happened in the Middle East, China, and Australia. The overall aim of the present work is therefore to obtain and analyse the relevant information pertaining to the fire-safe use of CLT and ACPs in the built environment. In order to achieve this aim, we have employed some of the relevant analytical techniques to gather the required data. Here, we have also endeavoured to seek analytical correlations, if any, between the experimental results, with a view to gauging the materials’ behaviours in real fire scenarios.
4. Conclusions
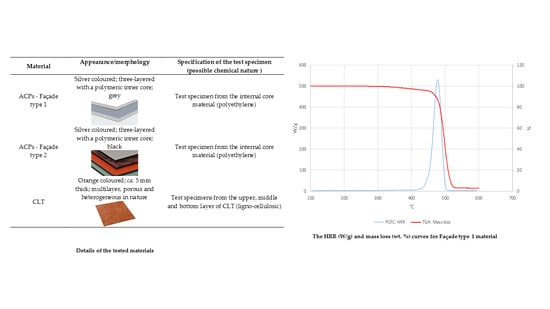
The results obtained in the current study provide an essential for construction sector information in relation to thermal degradation and combustion of two common materials, ACPs and CLT. The lack of such information had led to tragedies linked to fires involving tall building. Among all the materials tested in this work, the CLT demonstrated better fire-retardant attributes compared to ACPs and thus is more fire safe when used for pre-fabricated houses. In contrast, the inner core of the ACP façade materials is highly likely to contribute to the fire load/growth in a real fire scenario. This paper highlights significant fire hazards linked the use of ACPs for pre-fabricated houses around the world. The bench-scale tests revealed a drastic difference in the behaviour of different layers of ACPs. These results highlight the need of properly fire-proofing the internal core of ACPs, for example by substituting with polymers containing reactive fire retardant groups.
The results of TGA can only be treated as a first port-of-call in gauging the flammability aspects of the materials under investigation. However, the induction temperatures, slopes of the degradative steps, and the char residues obtained from the thermograms have provided useful inferences as to the behaviours of the materials under heat/fire conditions. The PCFC runs, on the other hand, have furnished a better overall profile, especially, relating to the combustion behaviours of ACPs and CLT materials in the present study.
It is significant to note the reasonable correlations found between the thermograms and HRR curves obtained through TGA and PFCF runs. However, a great difference in the char yields measured by the two techniques was observed. Depending on the tested material, the char yield left after the PCFC can be 4–20 times higher than the one obtained in TGA run.