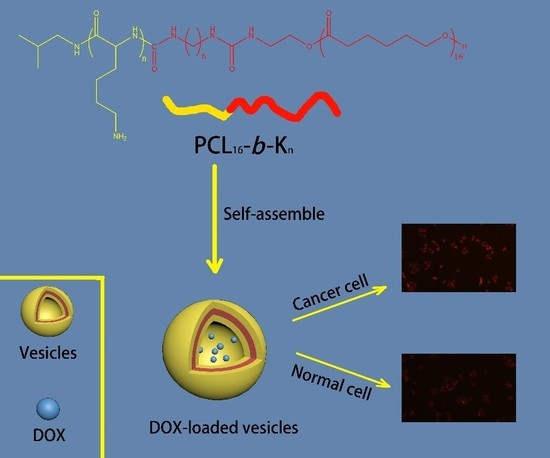
Polycaprolactone-Based Mimetic Antimicrobial Peptide Copolymers Vesicles as an Effective Drug-Carrier for Cancer Therapy
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of PCL16-b-Kn Copolymer Vesicles
2.3. Critical Vesiculation Concentration (CVC) Test
2.4. In Vitro DOXencapulation and Release
2.5. Characterization
2.6. Intracellular Release of DOX-Loaded PCL16-b-K20 Nanoparticles
3. Results and Discussion
3.1. Synthesis of PCL16-b-Kn Copolymers
3.2. Self-Assembly of PCL16-b-Kn Copolymers
3.3. In Vitro DOX Encapulation and Release
3.4. Intracellular Drug Release of DOX-loaded PCL16-b-K20 Vesicles
3.5. CCK-8 Test of DOX-Loaded PCL16-b-K20 Vesicles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, T.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Perez-Herrero, E.; Fernandez-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabner, B.A.; Roberts, T.G. Timeline—Chemotherapy and the war on cancer. Nat. Rev. Cancer. 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Amaral, M.H.; Conceição, J.; Sousa Lobo, J.M. Nanotechnological carriers for cancer chemotherapy: The state of the Art. Colloids Surf. B. 2015, 126, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.G.; Lv, L.; Yang, K. Chemotherapy targeting cancer stem cells. Am. J. Cancer Res. 2015, 5, 880–893. [Google Scholar] [PubMed]
- Kim, C.J.; Kurauchi, S.; Uebayashi, T.; Fujisaki, A.; Kimura, S. Morphology change from nanotube to vesicle and monolayer/bilayer alteration by amphiphilic block polypeptides having aromatic groups at C terminal. Bull. Chem. Soc. Jpn. 2017, 90, 568–573. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, X.; Lund, R.; Downing, K.H.; Balsara, N.P.; Zuckermann, R.N. Self-Assembly of crystalline nanotubes from monodisperse amphiphilic diblockcopolypeptoid tiles. Proc. Natl. Acad. Sci. USA 2016, 113, 3954–3959. [Google Scholar] [CrossRef]
- Burgess, N.C.; Sharp, T.H.; Thomas, F.; Wood, C.W.; Thomson, A.R.; Zaccai, N.R.; Brady, R.L.; Serpell, L.C.; Woolfson, D.N. Modular design of self-assembling peptide-based nanotubes. J. Am. Chem. Soc. 2015, 137, 10554–10562. [Google Scholar] [CrossRef]
- Song, Z.Y.; Kim, H.J.; Ba, X.C.; Baumgartner, R.; Lee, J.S.; Tang, H.Y.; Leal, C.; Cheng, J.J. Polypeptide vesicles with densely packed multilayer membranes. Soft Matter. 2015, 11, 4091–4098. [Google Scholar] [CrossRef]
- Song, J.B.; Yang, X.Y.; Jacobson, O.; Lin, L.S.; Huang, P.; Niu, P.; Ma, Q.J.; Chen, X.Y. Sequential drug release and enhanced photothermal and photoacoustic effect of hybrid reduced graphene oxide-loaded ultrasmall gold nanorod vesicles for cancer therapy. ACS Nano 2015, 9, 9199–9209. [Google Scholar] [CrossRef]
- Ghanbarzadeh, S.; Khorrami, A.; Arami, S. Nonionic surfactant-based vesicular system for transdermal drug delivery. Drug Deliv. 2015, 22, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Toughraï, S.; Malinova, V.; Masciadri, R.; Menon, S.; Tanner, P.; Palivan, C.; Bruns, N.; Meier, W. Reduction-sensitive amphiphilic triblock copolymers self-assemble into stimuli-responsive micelles for drug delivery(a). Macromol. Biosci. 2015, 15, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.B.; Hudson, Z.M.; Winnik, M.A.; Manners, I. Multidimensional hierarchical self-assembly of amphiphilic cylindrical block comicelles. Science 2015, 347, 1329–13332. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.M.P.; Liljeström, V.; Posocco, P.; Laurini, E.; Pricl, S.; Kostiainen, M.A.; Smith, D.K. Emergence of highly-ordered hierarchical nanoscale aggregates on electrostatic binding of self-assembled multivalent (SAMul) cationic micelles with polyanionic heparin. J. Mater. Chem. B 2017, 5, 341–347. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, P.Y.; Xie, M.L.; Wu, C.F.; Luo, Y.F.; Wang, W.T.; Jiang, J.; Liang, G.L. Cell environment-differentiated self-assembly of nanofibers. J. Am. Chem. Soc. 2016, 138, 11128–11131. [Google Scholar] [CrossRef]
- Moore, A.N.; Hartgerink, J.D. Self-Assembling multidomain peptide nanofibers for delivery of bioactive molecules and tissue regeneration. Acc. Chem. Res. 2017, 50, 714–722. [Google Scholar] [CrossRef]
- Fisusi, F.A.; Notman, R.; Granger, L.A.; Malkinson, J.P.; Schatzlein, A.G.; Uchegbu, I.F. T-shaped peptide amphiphiles self assemble into nanofiber networks. Pharm. Nanotechnol. 2017, 5, 215–219. [Google Scholar] [CrossRef]
- Shi, Z.K.; Wei, Y.H.; Zhu, C.H.; Sun, J.; Li, Z.B. Crystallization-Driven two-dimensional nanosheet from hierarchical self-assembly of polypeptoid-based diblock copolymers. Macromolecules 2018, 51, 6344–6351. [Google Scholar] [CrossRef]
- Flood, D.; Proulx, C.; Robertson, E.J.; Battigelli, A.; Wang, S.; Schwartzberg, A.M.; Zuckermann, R.N. Improved chemical and mechanical stability of peptoidnanosheets by photo-crosslinking the hydrophobic core. Chem. Commun. 2016, 52, 4753–4756. [Google Scholar] [CrossRef]
- Sun, T.M.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.X.; Xia, Y.N. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Xu, X.Y.; Ho, W.; Zhang, X.Q.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Park, K. New horizons in drug and gene delivery—ICRS 2016. J. Control. Release 2017, 257, 1. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Jain, S.; Mahajan, S.C. Nanomedicines based drug delivery systems for anti-cancer targeting and treatment. Curr. Drug Deliv. 2015, 12, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.M.; Cui, H.Q.; Shi, R.W.; Guo, J.N.; Wang, B.; Xu, Y.; Ding, Y.Y.; Mao, H.L.; Yan, F. Antimicrobial anionic polymers: The effect of cations. Eur. Polym. J. 2018, 107, 181–188. [Google Scholar] [CrossRef]
- Engler, A.C.; Wiradharma, N.; Ong, Z.Y.; Coady, D.J.; Hedrick, J.L.; Yang, Y.Y. Emerging trends in macromolecular antimicrobials to fight multi-drug-resistant infections. Nano Today 2012, 7, 201–222. [Google Scholar] [CrossRef]
- Bechinger, B.; Gorr, S.U. Antimicrobial peptides: Mechanisms of action and resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef]
- Su, X.K.; Zhou, X.Y.; Tan, Z.Z.; Zhou, C.C. Highly efficient antibacterial diblock copolypeptides based on lysine and phenylalanine. Biopolymers 2017, 107, e23041. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Su, X.K.; Tan, Z.Z.; Zhou, C.C. Synthesis of triblock amphiphilic copolypeptides with excellent antibacterial activity. Eur. Polym. J. 2018, 106, 175–181. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Su, X.K.; Zhou, C.C. Preparation of diblock amphiphilic polypeptide nanoparticles for medical applications. Eur. Polym. J. 2018, 100, 132–136. [Google Scholar] [CrossRef]
- Punia, A.; Debata, P.R.; Banerjee, P.; Yang, N.L. Structure-property relationships of antibacterial amphiphilic polymers derived from 2-aminoethyl acrylate. RSC Adv. 2015, 5, 95300–95306. [Google Scholar] [CrossRef]
- Chakraborty, S.; Liu, R.H.; Hayouka, Z.; Chen, X.Y.; Ehrhardt, J.; Lu, Q.; Burke, E.; Yang, Y.Q.; Weisblum, B.; Wong, G.C.L.; et al. Ternary Nylon-3 copolymers as host-defense peptide mimics: Beyond hydrophobic and cationic subunits. J. Am. Chem. Soc. 2014, 136, 14530–14535. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Chen, X.Y.; Chakraborty, S.; Lemke, J.J.; Hayouka, Z.; Chow, C.; Welch, R.A.; Weisblum, B.; Masters, K.S.; Gellman, S.H. Tuning the biological activity profile of antibacterial polymers via subunit substitution pattern. J. Am. Chem. Soc. 2014, 136, 4410–4418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, CC.; Wang, MZ.; Zou, KD.; Chen, J.; Zhu, YQ.; Du, JZ. Antibacterial polypeptide-grafted chitosan-based nanocapsules as an “armed” carrier of anticancer and antiepileptic drugs. ACS Macro Lett. 2013, 2, 1021–1025. [Google Scholar] [CrossRef]
- Zhou, X.Y.; He, J.; Zhou, C.C. Strategies from nature: Polycaprolactone-based mimetic antimicrobial peptide block copolymers with low cytotoxicity and excellent antibacterial efficiency. Polym. Chem. 2019, 10, 945–953. [Google Scholar] [CrossRef]
- Liu, Q.M.; Song, L.W.; Chen, S.A.; Gao, J.Y.; Zhao, P.Y.; Du, J.Z. A superparamagnetic polymersome with extremely high T-2 relaxivity for MRI and cancer-targeted drug delivery. Biomaterials 2017, 114, 23–33. [Google Scholar] [CrossRef]
- Oliveira, H.; Pérez-Andrés, E.; Thevenot, J.; Sandre, O.; Berra, E.; Lecommandoux, S. Magnetic field triggered drug release from polymersomes for cancer therapeutics. J. Control. Release 2013, 169, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, Q.M.; Xiao, J.G.; Du, J.Z. EpCAM-antibody-labeled noncytotoxic polymer vesicles for cancer stem cells-targeted delivery of anticancer drug and siRNA. Biomacromolecules 2015, 16, 1695. [Google Scholar] [CrossRef]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Goncalves, L.M.D. Chitosan nanoparticles as a mucoadhesive drug delivery system for ocular administration. Mar. Drugs 2017, 12, 370. [Google Scholar] [CrossRef]
- Gibis, M.; Ruedt, C.; Weiss, J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 3, 217–223. [Google Scholar]
- Iwasaki, T.; Ishibashi, J.; Tanaka, H.; Sato, M.; Asaoka, A.; Taylor, D.; Yamakawa, M. Selective cancer cell cytotoxicity of enantiomeric 9-mer peptides derived from beetle defensins depends on negatively charged phosphatidylserine on the cell surface. Peptides 2009, 30, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Jin, S.M.; Han, E.H.; Ko, E.; Ahn, M.; Bang, W.Y.; Bang, J.K.; Lee, E. Structure-Dependent antimicrobial theranostic functions of self-assembled short peptide nanoagents. Biomacromolecules 2017, 11, 3600–3610. [Google Scholar] [CrossRef]







| Models | Formula | r2 |
|---|---|---|
| Zero-Order | 0.6504 | |
| First-Order | 0.9942 | |
| Hixson-Crowell | 0.9925 | |
| Higuchi | 0.9461 | |
| Korsmeyer-Peppas | 0.9408 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Y.; Zhou, X.; He, J.; Zhou, C. Polycaprolactone-Based Mimetic Antimicrobial Peptide Copolymers Vesicles as an Effective Drug-Carrier for Cancer Therapy. Polymers 2019, 11, 1783. https://doi.org/10.3390/polym11111783
Qian Y, Zhou X, He J, Zhou C. Polycaprolactone-Based Mimetic Antimicrobial Peptide Copolymers Vesicles as an Effective Drug-Carrier for Cancer Therapy. Polymers. 2019; 11(11):1783. https://doi.org/10.3390/polym11111783
Chicago/Turabian StyleQian, Yusheng, Xinyu Zhou, Jing He, and Chuncai Zhou. 2019. "Polycaprolactone-Based Mimetic Antimicrobial Peptide Copolymers Vesicles as an Effective Drug-Carrier for Cancer Therapy" Polymers 11, no. 11: 1783. https://doi.org/10.3390/polym11111783