Construction of Antifouling Membrane Surfaces through Layer-by-Layer Self-Assembly of Lignosulfonate and Polyethyleneimine
Abstract
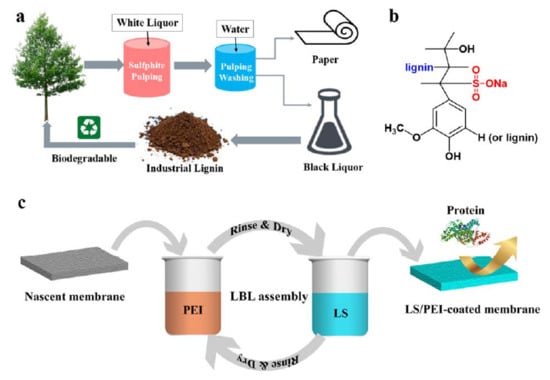
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication and Characterization of LS/PEI Multilayers on PSf Membranes
2.2.1. Preparation of PSf Membranes
2.2.2. LbL Deposition
2.2.3. Characterization
2.3. Antifouling Performances of the Membranes
2.3.1. Static Protein Adsorption
2.3.2. Dynamic Antifouling Test
3. Results and Discussion
3.1. Formation of LS/PEI Multilayers on PSf Membranes
3.2. Surface Morphologies of LS/PEI Multilayers on PSf Membranes
3.3. Antifouling Performances of the Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, C.; Li, H.N.; Du, Y.; Ma, M.Q.; Xu, Z.K. CuSO4/H2O2-Triggered Polydopamine/Poly(sulfobetaine methacrylate) Coatings for Antifouling Membrane Surfaces. Langmuir 2017, 33, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, R.N.; Liu, Y.N.; He, M.R.; Su, Y.L.; Gao, C.J.; Jiang, Z.Y. Antifouling membrane surface construction: Chemistry plays a critical role. J. Membr. Sci. 2018, 551, 145–171. [Google Scholar] [CrossRef]
- Chen, W.; Chen, W.J.; Su, Y.L.; Peng, J.M.; Dong, Y.N.; Zhao, X.T.; Jiang, Z.Y. Engineering a Robust, Versatile Amphiphilic Membrane Surface Through Forced Surface Segregation for Ultralow Flux-Decline. Adv. Funct. Mater. 2011, 21, 191–198. [Google Scholar] [CrossRef]
- Shi, Q.; Su, Y.L.; Zhao, W.; Li, C.; Hu, Y.H.; Jiang, Z.Y.; Zhu, S.P. Zwitterionic polyethersulfone ultrafiltration membrane with superior antifouling property. J. Membr. Sci. 2008, 319, 271–278. [Google Scholar] [CrossRef]
- Yi, Z.; Zhu, L.P.; Cheng, L.; Zhu, B.K.; Xu, Y.Y. A readily modified polyethersulfone with amino-substituted groups: Its amphiphilic copolymer synthesis and membrane application. Polymers 2012, 53, 350–358. [Google Scholar] [CrossRef]
- Venault, A.; Chang, Y.; Wang, D.-M.; Bouyer, D.; Higuchi, A.; Lai, J.-Y. PEGylation of anti-biofouling polysulfone membranes via liquid- and vapor-induced phase separation processing. J. Membr. Sci. 2012, 403–404, 47–57. [Google Scholar] [CrossRef]
- Sinha, M.K.; Purkait, M.K. Preparation of fouling resistant PSF flat sheet UF membrane using amphiphilic polyurethane macromolecules. Desalination 2015, 355, 155–168. [Google Scholar] [CrossRef]
- Khan, A.; Sherazi, T.A.; Khan, Y.; Li, S.; Naqvi, S.A.R.; Cui, Z. Fabrication and characterization of polysulfone/modified nanocarbon black composite antifouling ultrafiltration membranes. J. Membr. Sci. 2018, 554, 71–82. [Google Scholar] [CrossRef]
- Li, D.; Niu, X.; Yang, S.; Chen, Y.; Ran, F. Thermo-responsive polysulfone membranes with good anti-fouling property modified by grafting random copolymers via surface-initiated eATRP. Sep. Purif. Technol. 2018, 206, 166–176. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymers 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Ren, P.-F.; Yang, H.-C.; Xu, Z.-K. Fabrication of antifouling membrane surface by poly(sulfobetaine methacrylate)/polydopamine co-deposition. J. Membr. Sci. 2014, 466, 18–25. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Su, Y.; Sun, Q.; Wang, Y.; Jiang, Z. Enhancing the antifouling property of polyethersulfone ultrafiltration membranes through surface adsorption-crosslinking of poly(vinyl alcohol). J. Membr. Sci. 2007, 300, 71–78. [Google Scholar] [CrossRef]
- Koehler, J.A.; Ulbricht, M.; Belfort, G. Intermolecular Forces between a Protein and a Hydrophilic Modified Polysulfone Film with Relevance to Filtration. Langmuir 2000, 16, 10419–10427. [Google Scholar] [CrossRef]
- Ren, P.F.; Yang, H.C.; Liang, H.Q.; Xu, X.L.; Wan, L.S.; Xu, Z.K. Highly Stable, Protein-Resistant Surfaces via the Layer-by-Layer Assembly of Poly(sulfobetaine methacrylate) and Tannic Acid. Langmuir 2015, 31, 5851–5858. [Google Scholar] [CrossRef]
- Xu, G.-R.; Wang, S.-H.; Zhao, H.-L.; Wu, S.-B.; Xu, J.-M.; Li, L.; Liu, X.-Y. Layer-by-layer (LBL) assembly technology as promising strategy for tailoring pressure-driven desalination membranes. J. Membr. Sci. 2015, 493, 428–443. [Google Scholar] [CrossRef]
- Joseph, N.; Ahmadiannamini, P.; Hoogenboom, R.; Vankelecom, I.F.J. Layer-by-layer preparation of polyelectrolyte multilayer membranes for separation. Polym. Chem. 2014, 5, 1817–1831. [Google Scholar] [CrossRef]
- Xu, G.-R.; Wang, J.-N.; Li, C.-J. Strategies for improving the performance of the polyamide thin film composite (PA-TFC) reverse osmosis (RO) membranes: Surface modifications and nanoparticles incorporations. Desalination 2013, 328, 83–100. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, C.; van der Bruggen, B. Technology-driven layer-by-layer assembly of a membrane for selective separation of monovalent anions and antifouling. Nanoscale 2019, 11, 2264–2274. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, S.; Cao, H.; Li, Y.; Van der Bruggen, B. Layer-by-layer assembly of anion exchange membrane by electrodeposition of polyelectrolytes for improved antifouling performance. J. Membr. Sci. 2018, 558, 1–8. [Google Scholar] [CrossRef]
- He, M.; Wang, Q.; Zhao, W.F.; Zhao, C.S. A substrate-independent ultrathin hydrogel film as an antifouling and antibacterial layer for a microfiltration membrane anchored via a layer-by-layer thiol-ene click reaction. J. Mater. Chem. B 2018, 6, 3904–3913. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, L.; Zhang, X.; Chen, S.; Zhang, M.; Zhao, W.; Sun, S.; Zhao, C. Integrating zwitterionic polymer and Ag nanoparticles on polymeric membrane surface to prepare antifouling and bactericidal surface via Schiff-based layer-by-layer assembly. J. Colloid Interface Sci. 2018, 510, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Therien-Aubin, H.; Wong, M.C.Y.; Hoek, E.M.V.; Ober, C.K. Improved antifouling properties of polymer membranes using a ‘layer-by-layer’ mediated method. J. Mater. Chem. B 2013, 1, 5651–5658. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhan, J.; Pan, H.; Wang, W.; Tang, G.; Song, L.; Hu, Y. Effect of Fully Biobased Coatings Constructed via Layer-by-Layer Assembly of Chitosan and Lignosulfonate on the Thermal, Flame Retardant, and Mechanical Properties of Flexible Polyurethane Foam. ACS Sustain. Chem. Eng. 2016, 4, 1431–1438. [Google Scholar] [CrossRef]
- Gu, L.; Cui, B.; Wu, Q.-Y.; Yu, H. Bio-based polyurethanes with shape memory behavior at body temperature: Effect of different chain extenders. RSC Adv. 2016, 6, 17888–17895. [Google Scholar] [CrossRef]
- Chaleawlert-umpon, S.; Berthold, T.; Wang, X.; Antonietti, M.; Liedel, C. Kraft Lignin as Electrode Material for Sustainable Electrochemical Energy Storage. Adv. Mater. Interfaces 2017, 4, 1700698. [Google Scholar] [CrossRef]
- Ding, J. Epoxidation Modification of Renewable Lignin to Improve the Corrosion Performance of Epoxy Coating. Int. J. Electrochem. Sci. 2016, 11, 6256–6265. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Pan, Y.H.; Jin, W.Z.; Xu, J.M.; Lao, K.K.; Gu, L. Preparation of Sodium Lignin Sulfonate Modified Polysulfone Membranes and Their Use as Supports for Forward Osmosis Membranes. Acta Polym. Sin. 2017, 5, 851–857. [Google Scholar]
- Xia, K.; Ouyang, Q.; Chen, Y.; Wang, X.; Qian, X.; Wang, L. Preparation and Characterization of Lignosulfonate–Acrylonitrile Copolymer as a Novel Carbon Fiber Precursor. ACS Sustain. Chem. Eng. 2016, 4, 159–168. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Lopez Garcia, I.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kelley, S.S.; Venditti, R.A. Lignin-Based Thermoplastic Materials. Chemsuschem 2016, 9, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, L. Antimicrobial and antioxidant surface modification of cellulose fibers using layer-by-layer deposition of chitosan and lignosulfonates. Carbohydrate. Polymers 2015, 124, 35–42. [Google Scholar]
- Ge, Y.; Li, Z. Application of Lignin and Its Derivatives in Adsorption of Heavy Metal Ions in Water: A Review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Pang, B.; Yan, J.P.; Yao, L.; Liu, H.; Guan, J.; Wang, H.S.; Liu, H.Z. Preparation and characterization of antibacterial paper coated with sodium lignosulfonate stabilized ZnO nanoparticles. RSC Adv. 2016, 6, 9753–9759. [Google Scholar] [CrossRef]
- Deng, Y.H.; Wang, T.; Guo, Y.Q.; Qiu, X.Q.; Qian, Y. Layer-by-Layer Self-Assembled Films of a Lignin-based Polymer through Hydrogen Bonding. ACS Sustain. Chem. Eng. 2015, 3, 1215–1220. [Google Scholar] [CrossRef]
- Li, H.; Fu, S.Y.; Peng, L.C.; Zhan, H.Y. Surface modification of cellulose fibers with layer-by-layer self-assembly of lignosulfonate and polyelectrolyte: Effects on fibers wetting properties and paper strength. Cellulose 2012, 19, 533–546. [Google Scholar] [CrossRef]
- Liu, H.; Fu, S.Y.; Li, H.; Zhan, H.Y. Layer-by-layer assembly of lignosulfonates for hydrophilic surface modification. Ind. Crops Prod. 2009, 30, 287–291. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Fu, S.; Zhan, H. Surface hydrophobicity modification of cellulose fibers by layer-by-layer self-asssembly of lignosulfonates. Bioresources 2011, 6, 1681–1695. [Google Scholar]
- Chen, L.; Lu, D.L.; Ye, J.R.; Shen, Q. Fabrication, characterization and application of fully green superhydrophilic lignosulfonate multilayer films. Mater. Lett. 2017, 194, 217–219. [Google Scholar] [CrossRef]
- Chen, J.M.; Jiang, S.D.; Huang, Z.Q.; Tang, G.; Hu, Y. Self-assembly of hydroxyapatite with polyelectrolyte as a green flame retardant for poly(vinyl alcohol). J. Fire Sci. 2017, 35, 507–520. [Google Scholar] [CrossRef]
- Pan, Y.H.; Zhao, Q.Y.; Gu, L.; Wu, Q.Y. Thin film nanocomposite membranes based on imologite nanotubes blended substrates for forward osmosis desalination. Desalination 2017, 421, 160–168. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Q.-Y.; Liang, H.-Q.; Gu, L.; Xu, Z.-K. Preparation and characterization of cellulose triacetate membranes via thermally induced phase separation. J. Appl. Polym. Sci. 2017, 134, 44454. [Google Scholar] [CrossRef]
- Luo, H.; Shen, Q.; Ye, F.; Cheng, Y.-F.; Mezgebe, M.; Qin, R.-J. Structure and properties of layer-by-layer self-assembled chitosan/lignosulfonate multilayer film. Mater. Sci. Eng. C 2012, 32, 2001–2006. [Google Scholar] [CrossRef]
- Nyman, V.; Rose, G.; Ralston, J. The colloidal behaviour of kraft lignin and lignosulfonates. Colloids Surf. 1986, 21, 125–147. [Google Scholar] [CrossRef]
- Zhu, L.-J.; Song, H.-M.; Wang, G.; Zeng, Z.-X.; Zhao, C.-T.; Xue, Q.-J.; Guo, X.-P. Microstructures and performances of pegylated polysulfone membranes from an in situ synthesized solution via vapor induced phase separation approach. J. Colloid Interface Sci. 2018, 515, 152–159. [Google Scholar] [CrossRef]










| Samples | N1s | C1s | O1s | S2p |
|---|---|---|---|---|
| PSf | − | 79.08% | 16.24% | 4.67% |
| PSf-1BL | 3.03% | 72.85% | 20.08% | 3.65% |
| PSf-3BL | 5.33% | 68.98% | 21.63% | 3.27% |
| PSf-5BL | 6.12% | 68.67% | 22.12% | 3.09% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Xie, M.-Y.; Jin, Y.; He, M.; Xing, X.-Y.; Yu, Y.; Wu, Q.-Y. Construction of Antifouling Membrane Surfaces through Layer-by-Layer Self-Assembly of Lignosulfonate and Polyethyleneimine. Polymers 2019, 11, 1782. https://doi.org/10.3390/polym11111782
Gu L, Xie M-Y, Jin Y, He M, Xing X-Y, Yu Y, Wu Q-Y. Construction of Antifouling Membrane Surfaces through Layer-by-Layer Self-Assembly of Lignosulfonate and Polyethyleneimine. Polymers. 2019; 11(11):1782. https://doi.org/10.3390/polym11111782
Chicago/Turabian StyleGu, Lin, Meng-Yun Xie, Yu Jin, Min He, Xiao-Yan Xing, Yuan Yu, and Qing-Yun Wu. 2019. "Construction of Antifouling Membrane Surfaces through Layer-by-Layer Self-Assembly of Lignosulfonate and Polyethyleneimine" Polymers 11, no. 11: 1782. https://doi.org/10.3390/polym11111782