Effect of Nitrogen-Doped Graphene Oxide on the Aging Behavior of Nitrile–Butadiene Rubber
Abstract
:1. Introduction
2. Experimental
2.1. Materials
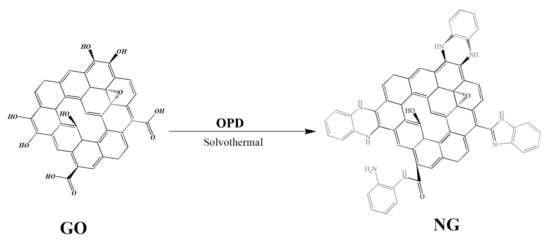
2.2. Preparation of Nitrogen-Doped Graphene Fillers
2.3. Preparation of NBR/NG Composites
2.4. Characterization
3. Results
3.1. Structure and Morphology of NG
3.2. Properties of NBR Composites
3.3. Reasons for Improving NBR Aging Resistance by an NG filler
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Cho, U.R. Enhanced thermo-physical properties of nitrile-butadiene rubber nanocomposites filled with simultaneously reduced and functionalized graphene oxide. Polym. Compos. 2018, 39, 3227–3235. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bandyopadhyay, S.; Ameta, R.; Mukhopadhyay, R.; Deuri, A.S. Application of FTIR in characterization of acrylonitrile-butadiene rubber (nitrile rubber). Polym. Test. 2007, 26, 38–41. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Xu, L.; Zhang, P. Investigation of aging behavior and mechanism of nitrile-butadiene rubber (NBR) in the accelerated thermal aging environment. Polym. Test. 2016, 54, 59–66. [Google Scholar] [CrossRef]
- Li, T.; Zhengren, S.; Xianru, H.; Ping, J.; Xiaobin, L.; Rui, Z.; Xin, W. Aging-Resistant Functionalized LDH–SAS/Nitrile-Butadiene Rubber Composites: Preparation and Study of Aging Kinetics/Anti-Aging Mechanism. Materials 2018, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, T.; Shi, Z.; Xin, W.; Qian, C. Thermal-oxidative aging behavior of nitrile-butadiene rubber/functional LDHs composites. Polym. Degrad. Stab. 2016, 133, 219–226. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Zhang, J.; Lu, X.; Yang, C.; Liu, T.; Wang, X.; Zhang, R. Influence of graphene/ferriferrous oxide hybrid particles on the properties of nitrile rubber. RSC Adv. 2016, 6, 91798–91805. [Google Scholar] [CrossRef]
- Jiang, P.; Yang, C.; He, X.; Rodrigues, A.M.; Zhang, R. Viscoelastic changes in chlorinated butyl rubber modified with graphene oxide. Iran. Polym. J. 2017, 26, 861–870. [Google Scholar] [CrossRef]
- Zhong, R.; Zhang, Z.; Zhao, H.; He, X.; Wang, X.; Zhang, R.J.M. Improving Thermo-Oxidative Stability of Nitrile Rubber Composites by Functional Graphene Oxide. Materials 2018, 11, 921. [Google Scholar] [CrossRef]
- Abdullah, H.N.; Woei, C.B.; Norkhairunnisa, M.; Umer, R.; Robiah, Y.; Abdul, R.S. Elastomeric Nanocomposite Based on Exfoliated Graphene Oxide and Its Characteristics without Vulcanization. J. Nanomater. 2017, 2017, 1–11. [Google Scholar]
- Li, Y.; Wang, Q.; Wang, T.; Pan, G. Preparation and tribological properties of graphene oxide/nitrile rubber nanocomposites. J. Mater. Sci. 2012, 47, 730–738. [Google Scholar] [CrossRef]
- Mensah, B.; Kim, S.; Arepalli, S.; Nah, C. A study of graphene oxide-reinforced rubber nanocomposite. J. Appl. Polym. Sci. 2014, 131, 590–600. [Google Scholar] [CrossRef]
- Wang, X.; Fang, K.; Xi, X.; Jia, D. Synthesis and anti-aging property in acrylonitrile-butadiene rubber of non-aromatic dendritic antioxidant with amine groups. J. Macromol. Sci. Part A. 2017, 54, 612–621. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, J.M.; Kim, J.D. Hydrothermal preparation of nitrogen-doped graphene sheets via hexamethylenetetramine for application as supercapacitor electrodes. Electrochim. Acta 2012, 85, 459–466. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, F.; Zhang, T.; Leng, K.; Zhang, L.; Yang, X.; Ma, Y.; Huang, Y.; Zhang, M.; Chen, Y. Synthesis and supercapacitor performance studies of N-doped graphene materials using o-phenylenediamine as the double-N precursor. Carbon 2013, 63, 508–516. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, F.; Yang, S.; Guo, T. A novel route synthesis of poly(ortho-phenylenediamine) fluffy microspheres self-assembled from nanospheres. Fibers Polym. 2011, 12, 997–1001. [Google Scholar] [CrossRef]
- Sestrem, R.H.; Ferreira, D.C.; Landers, R.; Temperini, M.L.A.; Nascimento, G.M.D. Synthesis and spectroscopic characterization of polymer and oligomers of ortho-phenylenediamine. Eur. Polym. J. 2010, 46, 484–493. [Google Scholar] [CrossRef]
- Varenik, M.; Nadiv, R.; Levy, I.; Vasilyev, G.; Regev, O. Breaking through the Solid/Liquid Processability Barrier: Thermal Conductivity and Rheology in Hybrid Graphene-Graphite Polymer Composites. ACS Appl. Mater. Interfaces 2017, 9, 7564. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A., Jr.; Carl, A.V. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Lin, Z.; Waller, G.; Liu, Y.; Liu, M.; Wong, C.P. Facile Synthesis of Nitrogen-Doped Graphene via Pyrolysis of Graphene Oxide and Urea, and its Electrocatalytic Activity toward the Oxygen-Reduction Reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Yan, J.; Xiao, Y.; Ning, G.; Wei, T.; Fan, Z. Facile and rapid synthesis of highly crumpled graphene sheets as high-performance electrodes for supercapacitors. RSC Adv. 2013, 3, 2566–2571. [Google Scholar] [CrossRef]
- Araby, S.; Meng, Q.; Zhang, L.; Kang, H.; Majewski, P.; Tang, Y.; Ma, J. Electrically and thermally conductive elastomer/graphene nanocomposites by solution mixing. Polymer 2014, 55, 201–210. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Wang, Y. A study on stress-softening of nitrile butadiene rubber reinforced by in situ zinc dimethacrylate. J. Reinf. Plast. Compos. 2012, 31, 705–716. [Google Scholar] [CrossRef]


















| Materials/phr a | KB-1 | KB-2 | NBR-NG-1 | NBR-NG-2 | NBR-NG-3 | NBR-NG-4 | NBR-RGO |
|---|---|---|---|---|---|---|---|
| NBR | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| ZnO | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Stearic acid | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sulfur | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| DM | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Antioxidant D | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| NG | 0 | 0 | 1 | 2 | 3 | 4 | 0 |
| RGO | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Character | Implication |
|---|---|
| Vr | Crosslinking density of NBR composites |
| V2 | Volume fraction of rubber phase in swelling NBR |
| χ | Interaction parameters between rubber and toluene |
| V | Molar volume of the solvent |
| m1 | Mass of the NBR composite sample before swelling |
| m2 | Mass of the NBR composite sample after swelling |
| m3 | Mass of the rubber phase |
| ρ | Density of NBR |
| ρs | Density of toluene |
| Sample | KB-1 | KB-2 | NBR | ||||
|---|---|---|---|---|---|---|---|
| NG-1 | NG-2 | NG-3 | NG-4 | RGO-3 | |||
| Stress at break (MPa) | 1.82 ± 0.23 | 2.19 ± 0.17 | 3.11 ± 0.32 | 3.24 ± 0.25 | 4.20 ± 0.18 | 4.02 ± 0.27 | 3.02 ± 0.38 |
| Elongation at break (%) | 663 ± 34 | 648 ± 31 | 651 ± 25 | 640 ± 19 | 620 ± 29 | 624 ± 36 | 554 ± 43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Li, T.; Wan, S.; Huang, X.; Cai, S.; He, X.; Zhang, R. Effect of Nitrogen-Doped Graphene Oxide on the Aging Behavior of Nitrile–Butadiene Rubber. Polymers 2019, 11, 1637. https://doi.org/10.3390/polym11101637
Chen S, Li T, Wan S, Huang X, Cai S, He X, Zhang R. Effect of Nitrogen-Doped Graphene Oxide on the Aging Behavior of Nitrile–Butadiene Rubber. Polymers. 2019; 11(10):1637. https://doi.org/10.3390/polym11101637
Chicago/Turabian StyleChen, Songbo, Tianxiang Li, Songhan Wan, Xing Huang, Shuwei Cai, Xianru He, and Rui Zhang. 2019. "Effect of Nitrogen-Doped Graphene Oxide on the Aging Behavior of Nitrile–Butadiene Rubber" Polymers 11, no. 10: 1637. https://doi.org/10.3390/polym11101637