Additive Manufacturing of Nerve Decellularized Extracellular Matrix-Contained Polyurethane Conduits for Peripheral Nerve Regeneration
Abstract
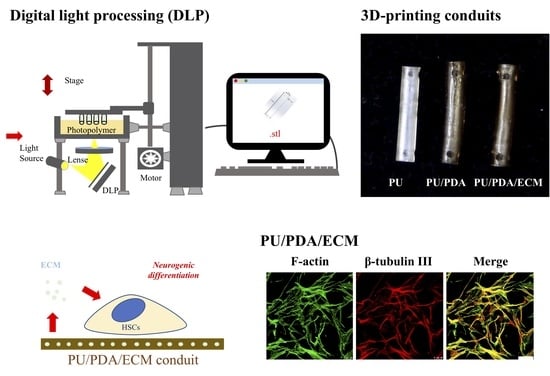
:1. Introduction
2. Materials and Methods
2.1. ECM Extraction
2.2. Preparation of Water-Based PU
2.3. Preparation of PU/PDA/ECM Matrix
2.4. Conduit Fabrication
2.5. Physical Properties of 3D-Printed Conduits
2.6. Biodegradation
2.7. Cytocompatibility of PU-Based Conduits Extracts
2.8. Enzyme-Linked Immunosorbent Assay
2.9. Cell Proliferation
2.10. Cell Differentiation
2.11. Immunofluorescence Staining
2.12. Statistical Analyses
3. Results and Discussion
3.1. Characterizations of PU/PDA/ECM Conduits
3.2. Cytotoxicity of PU/PDA/ECM Conduits
3.3. The Nerve-Regeneration-Promoting Growth Factor of Different Conduits
3.4. Cell Proliferation and Morphology
3.5. Neurogenic-Related Protein Expression
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheney, F.W.; Domino, K.; Caplan, R.A.; Posner, K.L. Nerve injury associated with anesthesia. Anesthesiology 1990, 90, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Kouyoumdjian, J.A. Peripheral nerve injuries: A retrospective survey of 456 cases. Muscle Nerve 2006, 34, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Li, C.Y.; Hsu, Y.M.; Tsai, C.H.; Tsai, F.J.; Tang, C.H.; Yang, J.S.; Wang, Z.H.; Yin, M.C. Oridonin, A natural diterpenoid, protected NGF-differentiated PC12 cells against MPP+- and kainic acid-induced injury. Food Chem. Toxicol. 2019, 133, 110765. [Google Scholar] [CrossRef] [PubMed]
- Tajdaran, K.; Gordon, T.; Wood, M.D.; Shoichet, M.S.; Borschel, G.H. A glial cell line-derived neurotrophic factor delivery system enhances nerve regeneration across acellular nerve allografts. Acta. Biomater. 2016, 29, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; Engel, E.A.; Krick, K.D.; Ju, A.; Meng, F.; et al. 3D printed anatomical nerve regeneration pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Lv, Y. Dual-delivery of VEGF and NGF by emulsion electrospun nanofibrous scaffold for peripheral nerve regeneration. Mater. Sci. Eng. C. Mater. Biol. Appl. 2018, 82, 253–264. [Google Scholar] [CrossRef]
- Singh, D.; Harding, A.J.; Albadawi, E.; Boissonade, F.M.; Haycock, J.W.; Claeyssens, F. Additive manufactured biodegradable poly(glycerol sebacate methacrylate) nerve guidance conduits. Acta. Biomater. 2018, 78, 48–63. [Google Scholar] [CrossRef]
- Ko, C.H.; Shie, M.Y.; Lin, J.H.; Chen, Y.W.; Yao, C.H.; Chen, Y.S. Biodegradable bisvinyl sulfonemethyl-crosslinked gelatin conduit promotes regeneration after peripheral nerve injury in adult rats. Sci. Rep. 2017, 7, 1062. [Google Scholar] [CrossRef]
- Chen, C.C.; Yu, J.; Ng, H.Y.; Lee, K.X.; Chen, C.C.; Chen, Y.S.; Shie, M.Y. The physicochemical properties of decellularized extracellular matrix-coated 3D printed poly(ε-caprolactone) nerve conduits for promoting Schwann cells proliferation and differentiation. Materials 2018, 11, 1665. [Google Scholar] [CrossRef]
- Maiti, B.; Díaz Díaz, D. 3D printed polymeric hydrogels for nerve regeneration. Polymers 2018, 10, 1041. [Google Scholar] [CrossRef]
- Kankala, R.K.; Xu, X.M.; Liu, C.G.; Chen, A.Z.; Wang, S.B. 3D-printing of microfibrous porous scaffolds based on hybrid approaches for bone tissue engineering. Polymers 2018, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Jubran, M.; Widenfalk, J. Repair of peripheral nerve transections with fibrin sealant containing neurotrophic factors. Exp. Neurol. 2003, 181, 204–212. [Google Scholar] [CrossRef]
- Wu, T.; Li, D.; Wang, Y.; Sun, B.; Li, D.; Li, D.; Morsi, Y.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Laminin-coated nerve guidance conduits based on poly(l-lactide-co-glycolide) fibers and yarns for promoting Schwann cells’ proliferation and migration. J. Mater. Chem. B 2017, 5, 3186–3194. [Google Scholar] [CrossRef]
- Toll, E.C.; Seifalian, A.M.; Birchall, M.A. The role of immunophilin ligands in nerve regeneration. Regen. Med. 2011, 6, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Daly, W.; Newland, B.; Yao, S.; Wang, W.C.; Chen, B.K.K.; Madigan, N.; Windebank, A.; Pandit, A. Improved axonal regeneration of transected spinal cord mediated by multichannel collagen conduits functionalized with neurotrophin-3 gene. Gene. Ther. 2013, 20, 1149–1157. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Shie, M.Y.; Lin, Y.H.; Lee, K.X.; Chen, Y.W. Effect of strontium substitution on the physicochemical properties and bone regeneration potential of 3D printed calcium silicate scaffolds. Int. J. Mol. Sci. 2019, 20, 2729. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chiu, Y.C.; Lin, Y.H.; Ho, C.C.; Shie, M.Y.; Chen, Y.W. 3D-printed bioactive calcium silicate/poly-ε-caprolactone bioscaffolds modified with biomimetic extracellular matrices for bone regeneration. Int. J. Mol. Sci. 2019, 20, 942. [Google Scholar] [CrossRef]
- Tsai, C.H.; Hung, C.H.; Kuo, C.N.; Chen, C.Y.; Peng, Y.N.; Shie, M.Y. Improved bioactivity of 3D printed porous titanium alloy scaffold with chitosan/magnesium-calcium silicate composite for orthopaedic applications. Materials 2019, 12, 203. [Google Scholar] [CrossRef]
- Huang, K.H.; Chen, Y.W.; Wang, C.Y.; Lin, Y.H.; Wu, Y.H.; Shie, M.Y.; Lin, C.P. Enhanced capability of BMP-2-loaded mesoporous calcium silicate scaffolds to induce odontogenic differentiation of human dental pulp cells. J. Endod. 2018, 44, 1677–1685. [Google Scholar] [CrossRef]
- Chen, J.Y.; Hwang, J.V.; Ao-Ieong, W.S.; Lin, Y.C.; Hsieh, Y.K.; Cheng, Y.L.; Wang, J. Study of physical and degradation properties of 3D-printed biodegradable, photocurable copolymers, PGSA-co-PEGDA and PGSA-co-PCLDA. Polymers 2018, 10, 1263. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Shen, Y.F.; Lee, K.X.; Lin, S.H.; Wu, Y.C.; Chen, Y.W. 3D printing of amino resin-based photosensitive materials on multi-parameter optimization design for vascular engineering applications. Polymers 2019, 11, 1394. [Google Scholar] [CrossRef] [PubMed]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, M.L.; Zhang, R.K.; Lu, G.; Zhao, C.Y.; Fu, F.; Sun, H.T.; Zhang, S.; Tu, Y.; Li, X.H. Collagen/heparin sulfate scaffolds fabricated by a 3D bioprinter improved mechanical properties and neurological function after spinal cord injury in rats. J. Biomed. Mater. Res. A 2017, 105, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Shen, Y.F.; Ho, C.C.; Yu, J.; Wu, Y.H.; Wang, K.; Shih, C.T.; Shie, M.Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C. Mater. Biol. Appl. 2018, 91, 679–687. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chuang, T.Y.; Chiang, W.H.; Chen, I.W.P.; Wang, K.; Shie, M.Y.; Chen, Y.W. The synergistic effects of graphene-contained 3D-printed calcium silicate/poly-ε-caprolactone scaffolds promote FGFR-induced osteogenic/angiogenic differentiation of mesenchymal stem cells. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 104, 109887. [Google Scholar] [CrossRef]
- Hong, S.; Kang, E.Y.; Byeon, J.; Jung, S.H.; Hwang, C. Embossed membranes with vascular patterns guide vascularization in a 3D tissue model. Polymers 2019, 11, 792. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef]
- Wang, W.; Lin, J.H.; Tsai, C.C.; Chuang, H.C.; Ho, C.Y.; Yao, C.H.; Chen, Y.S. Biodegradable glutaraldehyde-crosslinked casein conduit promotes regeneration after peripheral nerve injury in Adult Rats. Macromol. Biosci. 2011, 11, 914–926. [Google Scholar] [CrossRef]
- Huang, Y.C.; Huang, C.C.; Huang, Y.Y.; Chen, K.S. Surface modification and characterization of chitosan or PLGA membrane with laminin by chemical and oxygen plasma treatment for neural regeneration. J. Biomed. Mater. Res. A 2007, 82, 842–851. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, Z.; Li, D.; Wu, T.; Zheng, H.; Liu, J.; Wang, G.; Yu, Y.; Mo, X. Polypyrrole-coated poly(l-lactic acid-co-ε-caprolactone)/silk fibroin nanofibrous nerve guidance conduit induced nerve regeneration in rat. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 94, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, D.; Shibayama, M.; Lynam, D.A.; Wolf, K.J.; Kubota, G.; Koffler, J.Y.; Tuszynski, M.H.; Campana, W.M.; Sakamoto, J.S. Peripheral nerve growth within a hydrogel microchannel scaffold supported by a kink-resistant conduit. J. Biomed. Mater. Res. A 2017, 105, 3392–3399. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Kaplan, D.L.; Kim, H.W.; Kundu, S.C. Prospects of peripheral nerve tissue engineering using nerve guide conduits based on silk fibroin protein and other biopolymers. I. Mater. Rev. 2017, 62, 367–391. [Google Scholar] [CrossRef]
- Heng, B.C.; Gong, T.; Wang, S.; Lim, L.W.; Wu, W.; Zhang, C. Decellularized matrix derived from neural differentiation of embryonic stem cells enhances the neurogenic potential of dental follicle stem cells. J. Endod. 2017, 43, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, S.; Wang, G.; Su, M.; Song, L.; Chen, J.; Fan, S.; Lin, X. Preparation of decellularized biphasic hierarchical myotendinous junction extracellular matrix for muscle regeneration. Acta. Biomater. 2018, 68, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yang, S.; Kim, M.; Kim, G. A scaffold with a bio-mimetically designed micro/nano-fibrous structure using decellularized extracellular matrix. RSC Adv. 2016, 6, 29697–29706. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Luo, B.; Chen, X.; Wen, W.; Zhou, C. Icariin immobilized electrospinning poly(l-lactide) fibrous membranes via polydopamine adhesive coating with enhanced cytocompatibility and osteogenic activity. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 79, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.G.; Hwang, K.J.; Lee, D.; Kim, T.; Kim, N. Enhanced mechanical properties of self-polymerized polydopamine-coated recycled PLA filament used in 3D printing. Appl. Surf. Sci. 2018, 441, 381–387. [Google Scholar] [CrossRef]
- Bidgoli, M.R.; Alemzadeh, I.; Tamjid, E.; Khafaji, M.; Vossoughi, M. Fabrication of hierarchically porous silk fibroin-bioactive glass composite scaffold via indirect 3D printing: Effect of particle size on physico-mechanical properties and in vitro cellular behavior. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 103, 109688. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chen, Y.W.; Wang, K.; Shie, M.Y. Enhanced adhesion and differentiation of human mesenchymal stem cell inside apatite-mineralized/poly(dopamine)-coated poly(ε-caprolactone) scaffolds by stereolithography. J. Mater. Chem. B 2016, 4, 6307–6315. [Google Scholar] [CrossRef]
- Lin, C.C.; Fu, S.J. Osteogenesis of human adipose-derived stem cells on poly(dopamine)-coated electrospun poly(lactic acid) fiber mats. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016, 58, 254–263. [Google Scholar] [CrossRef]
- Topp, K.S.; Boyd, B.S. Structure and biomechanics of peripheral nerves: Nerve responses to physical stresses and implications for physical therapist practice. Phys. Ther. 2006, 86, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.M.; Abliz, E.; Wachrathit, K.; Krauthamer, V.; Shah, S.B. Biomechanical and functional variation in rat sciatic nerve following cuff electrode implantation. J. Neuroeng. Rehabil. 2014, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Solouk, A.; Mirzadeh, H.; Seifalian, A.M. Electroconductive polyurethane/graphene nanocomposite for biomedical applications. Compos. B 2019, 168, 421–431. [Google Scholar] [CrossRef]
- Meade, A.D.; Lyng, F.M.; Knief, P.; Byrne, H.J. Growth substrate induced functional changes elucidated by FTIR and Raman spectroscopy in in-vitro cultured human keratinocytes. Anal. Bioanal. Chem. 2007, 387, 1717–1728. [Google Scholar] [CrossRef]
- Morra, M. Biochemical modification of titanium surfaces: Peptides and ECM proteins. Eur. Cell Mater. 2006, 12, 1–15. [Google Scholar] [CrossRef]
- Bendszus, M.; Wessig, C.; Solymosi, L.; Reiners, K.; Koltzenburg, M. MRI of peripheral nerve degeneration and regeneration: Correlation with electrophysiology and histology. Exp. Neurol. 2004, 188, 171–177. [Google Scholar] [CrossRef]
- Shie, M.Y.; Chang, W.C.; Wei, L.J.; Huang, Y.H.; Chen, C.-H.; Shih, C.T.; Chen, Y.W.; Shen, Y.F. 3D printing of cytocompatible water-based light-cured polyurethane with hyaluronic acid for cartilage tissue engineering applications. Materials 2017, 10, 136. [Google Scholar] [CrossRef]
- Ding, Y.H.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurf. Biotribol. 2016, 2, 121–136. [Google Scholar] [CrossRef]
- Xi, Z.Y.; Xu, Y.Y.; Zhu, L.P.; Wang, Y.; Zhu, B.K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine). J. Membr. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Dinis, T.M.; Elia, R.; Vidal, G.; Dermigny, Q.; Denoeud, C.; Kaplan, D.L.; Egles, C.; Marin, F. 3D multi-channel bi-functionalized silk electrospun conduits for peripheral nerve regeneration. J. Mech. Behav. Biomed. Mater. 2015, 41, 43–55. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, Z.; Zhou, L.; Zhao, K.; Ma, X.; Cheng, G. Hydrophilic cell-derived extracellular matrix as a niche to promote adhesion and differentiation of neural progenitor cells. RSC Adv. 2017, 7, 45587–45594. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Namiki, J.; Shibata, S.; Mastuzaki, Y.; Okano, H. The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J. Histochem. Cytochem. 2010, 58, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, M.L.; Rao, A.J.; Demerdash, O.N.A.; Kalil, R.E. Expression of nestin by neural cells in the adult rat and human brain. PLoS ONE 2011, 6, e18535. [Google Scholar] [CrossRef] [PubMed]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-W.; Chen, C.-C.; Ng, H.Y.; Lou, C.-W.; Chen, Y.-S.; Shie, M.-Y. Additive Manufacturing of Nerve Decellularized Extracellular Matrix-Contained Polyurethane Conduits for Peripheral Nerve Regeneration. Polymers 2019, 11, 1612. https://doi.org/10.3390/polym11101612
Chen Y-W, Chen C-C, Ng HY, Lou C-W, Chen Y-S, Shie M-Y. Additive Manufacturing of Nerve Decellularized Extracellular Matrix-Contained Polyurethane Conduits for Peripheral Nerve Regeneration. Polymers. 2019; 11(10):1612. https://doi.org/10.3390/polym11101612
Chicago/Turabian StyleChen, Yi-Wen, Chien-Chang Chen, Hooi Yee Ng, Ching-Wen Lou, Yueh-Sheng Chen, and Ming-You Shie. 2019. "Additive Manufacturing of Nerve Decellularized Extracellular Matrix-Contained Polyurethane Conduits for Peripheral Nerve Regeneration" Polymers 11, no. 10: 1612. https://doi.org/10.3390/polym11101612