Surface Hydrophobic Modification of Microcrystalline Cellulose by Poly(methylhydro)siloxane Using Response Surface Methodology
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
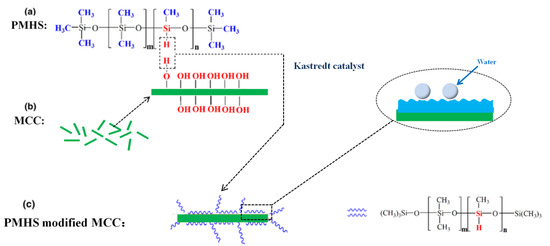
2.2. Surface Modification of MCC
2.3. Water Contact Angle (WCA) Measurement
2.4. Statistical Methods
2.5. X-ray Photoelectron Spectroscopy (XPS)
3. Results and Discussion
3.1. Hydrophobic Analysis
3.2. Experimental Design and Results
3.3. Analysis of Regression
3.4. Variable Analysis
3.4.1. Response Surface Methodology Analysis
3.4.2. Experimental Optimization Verification
3.5. XPS Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Lli, J.; Deng, X.; Jin, X.; Nie, S. Study on the performance of waste paper fiber/microcrystalline cellulose reinforced PHBV composite. J. Funct. Mater. 2014, 49, 8097–8101. [Google Scholar]
- Grunert, M.; Winter, W.T. Nanocomposites of cellulose acetate butyrate reinforced with cellulosenanocrystals. J. Polym. Environ. 2002, 10, 27–30. [Google Scholar] [CrossRef]
- Fu, J.; Tian, Y.; Tao, J. Surface groups and their modification of nano-microcrystalline cellulose. China Pulp Pap. 2008, 37, 50–59. [Google Scholar]
- Matuana, L.M.; Woodhams, R.T.; Balatinecz, J.J.; Park, C.B. Influence of Interfacial Interactions on the Properties of PVC/Ce llulose Fiber Composites. Polym. Compos. 1998, 19, 446–455. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Pechyen, C. Microcrystalline-cellulose and polypropylene based composite: A simple, selective and effective material for microwavable packaging. Carbohydr. Polym. 2016, 142, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, N.I.; Samat, N.; Anuar, H.; Zainuddin, N. Mechanical properties and failure modes of recycled polypropylene/microcrystalline cellulose composites. Mater. Des. 2015, 69, 114–123. [Google Scholar] [CrossRef]
- Spoljaric, S.; Genovese, A.; Shanks, R.A. Polypropylene-microcrystalline cellulose composites with enhanced compatibility and properties. Compos. A Appl. Sci. Manuf. 2009, 40, 791–799. [Google Scholar] [CrossRef]
- Głowin’ska, E.; Datta, J. Structure, morphology and mechanical behaviour of novel bio-based polyurethane composites with microcrystalline cellulose. Cellulose 2015, 22, 2471–2481. [Google Scholar] [CrossRef]
- Głowin’ska, E.; Datta, J. Bio-polyetherurethane composites with high content of natural ingredients: Hydroxylated soybean oil based polyol, bio glycol and microcrystalline cellulose. Cellulose 2016, 23, 581–592. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, Y.; Ge, X.; Li, M.; Li, X.; Cho, U.R. Graft copolymers of microcrystalline cellulose as reinforcing agent for elastomers based on natural rubber. J. Appl. Polym. Sci. 2016, 133, 43087. [Google Scholar] [CrossRef]
- Deng, F.; Ge, X.; Zhang, Y.; Li, M.C.; Cho, U.R. Synthesis and characterization of microcrystalline cellulose-graft-poly(methyl methacrylate) copolymers and their application as rubber reinforcements. J. Appl. Polym. Sci. 2015, 132, 42666. [Google Scholar] [CrossRef]
- Filson, P.B.; Dawson-Andoh, B.E. Sono-chemical preparation of cellulose nanocrystals from lignocelluloses derives materials. Bioresour. Technol. 2009, 113, 1270–1275. [Google Scholar]
- Tang, L.; Ou, W.; Lin, W. Optimization of acid hydrolysis processing of nanocellulose crystal using response surface methodology. Chem. Ind. For. Prod. 2011, 31, 61–65. [Google Scholar]
- Bian, Y.; Zhu, Y.; Wang, H.; Bian, Z. Study on the process of cu-bivo4 visible light catalytic degradation of paracetamol pollutant by response surface method. South-North Water Transf. Water Conserv. Technol. 2014, 12, 22–25. [Google Scholar]
- Fan, B. Research progress of surface modification of inorganic powder by stearic acid in China. Plast. Addit. 2011, 5, 25–29. [Google Scholar]
- Hong, J.; Chen, T.; Tang, M.; Wang, S. Optimization of protein extraction process of leek seed by response surface method. J. Chin. Food Sci. 2013, 13, 89–96. [Google Scholar]
- Han, D.; Wang, J.; Zhu, X.; Xie, J. Response surface method to optimize the flash extraction process of gardenia polysaccharides. For. Chem. Ind. 2013, 33, 59–62. [Google Scholar]
- Yang, G.; Liu, W.; Hao, J. Study on radiation-grafted non-woven fabrics optimized by response surface method. Tianjin Text. Sci. Technol. 2015, 210, 10–12. [Google Scholar]
- Hong, D.; Sui, S.; Wu, W. Numerical simulation of effects of operating conditions on the molecular weight of polypropylene using a response surface method. J. Beijing Inst. Technol. 2015, 24, 254–259. [Google Scholar]
- Hu, X.; Liu, L.; Luo, Y.; Jia, D.; Cheng, L.; Hu, S. Preparation of superhydrophobic pmhs-sio2 coating by sol-gel method. Chin. J. Mater. Res. 2010, 24, 266–272. [Google Scholar]
- Zhang, X.; Shen, Z.; Fu, X.; Cai, C.; Zheng, Y. Effects of methyl containing hydrogenated silicone oil on properties of spray drying ammonium phosphate extinguishing powder. Chin. J. Process Eng. 2009, 09, 1222–1226. [Google Scholar]
- Bendahou, A.; Hajlane, A.; Dufresne, A.; Boufi, S.; Kmddami, H. Esterification and amidation for grafting long aliphatic chains on to cellulose nanocrystals: A comparative study. Res. Chem. Intermed. 2014, 41, 4293–4310. [Google Scholar] [CrossRef]
- Qiu, X.; Li, J. Analysis of XPS and Raman spectra of C(membrane)/Si(SiO2)/C(membrane). J. Southwest Norm. Univ. (Nat. Sci. Ed.) 2003, 28, 230–233. [Google Scholar]








| Factors | Code and Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A: concentration of PMHS/(%/1000) | 5 | 27.5 | 50 |
| B: reaction time/min | 10 | 20 | 30 |
| C: drying temperature/°C | 40 | 70 | 100 |
| Experiment Number | A | B | C | Experimental WCA (°) | Predicted WCA (°) |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 140.56 | 140.23 |
| 2 | 1 | 0 | 1 | 138.54 | 138.36 |
| 3 | 0 | 1 | −1 | 134.14 | 134.63 |
| 4 | −1 | −1 | 0 | 136.10 | 136.09 |
| 5 | 0 | 0 | 0 | 140.03 | 140.23 |
| 6 | 0 | 0 | 0 | 138.71 | 140.23 |
| 7 | 1 | −1 | 0 | 136.69 | 137.05 |
| 8 | 0 | −1 | 1 | 138.23 | 138.37 |
| 9 | 1 | 0 | −1 | 135.86 | 135.68 |
| 10 | 0 | −1 | −1 | 136.64 | 136.15 |
| 11 | 0 | 0 | 0 | 141.48 | 140.23 |
| 12 | −1 | 0 | −1 | 136.08 | 136.26 |
| 13 | 1 | 1 | 0 | 137.89 | 137.90 |
| 14 | −1 | 0 | 1 | 130.95 | 131.13 |
| 15 | 0 | 0 | 0 | 140.36 | 140.23 |
| 16 | 0 | 1 | 1 | 136.99 | 136.85 |
| 17 | −1 | 1 | 0 | 132.56 | 132.20 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 123.12 | 9 | 13.68 | 19.35 | 0.0004 |
| A | 22.09 | 1 | 22.09 | 31.26 | 0.0008 |
| B | 4.63 | 1 | 4.63 | 6.56 | 0.0375 |
| C | 4.95 | 1 | 4.95 | 7.00 | 0.0331 |
| AB | 5.62 | 1 | 5.62 | 7.95 | 0.0258 |
| AC | 15.23 | 1 | 15.23 | 21.55 | 0.0024 |
| A2 | 32.57 | 1 | 32.57 | 46.08 | 0.0003 |
| B2 | 11.28 | 1 | 11.28 | 15.95 | 0.0052 |
| C2 | 18.40 | 1 | 18.40 | 26.03 | 0.0014 |
| A2C | 5.95 | 1 | 5.95 | 8.42 | 0.0229 |
| Residual | 4.95 | 7 | 0.71 | ||
| Lack of Fit | 0.90 | 3 | 0.30 | 0.30 | 0.8269 |
| Pure Error | 4.05 | 4 | 1.01 | ||
| Cor Total | 128.07 | 16 |
| Samples | Element Concentration (%) | Atomic Ratio | Ratios of Functional Groups (C1s) (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | Si | O/C | C1 (C–C/C–H) | C2 (C–O) | C3 (C=O/O–C–O) | C4 (O–C=O) | C5 (C–Si) | |
| MCC | 55.37 | 44.23 | 0 | 0.80 | 4.96 | 68.50 | 8.52 | 18.02 | - |
| Modified MCC | 52.66 | 40.35 | 6.99 | 0.77 | 13.97 | 36.85 | 32.52 | 10.12 | 6.53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Cai, S.; Hou, Z.; Li, W.; Wang, Y.; Zhang, X.; Yang, W. Surface Hydrophobic Modification of Microcrystalline Cellulose by Poly(methylhydro)siloxane Using Response Surface Methodology. Polymers 2018, 10, 1335. https://doi.org/10.3390/polym10121335
Xie Y, Cai S, Hou Z, Li W, Wang Y, Zhang X, Yang W. Surface Hydrophobic Modification of Microcrystalline Cellulose by Poly(methylhydro)siloxane Using Response Surface Methodology. Polymers. 2018; 10(12):1335. https://doi.org/10.3390/polym10121335
Chicago/Turabian StyleXie, Yulin, Siquan Cai, Zhen Hou, Weihua Li, Yan Wang, Xinxiang Zhang, and Wenbin Yang. 2018. "Surface Hydrophobic Modification of Microcrystalline Cellulose by Poly(methylhydro)siloxane Using Response Surface Methodology" Polymers 10, no. 12: 1335. https://doi.org/10.3390/polym10121335