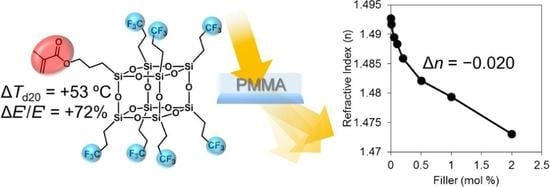
Fluoroalkyl POSS with Dual Functional Groups as a Molecular Filler for Lowering Refractive Indices and Improving Thermomechanical Properties of PMMA
Abstract
:1. Introduction
2. Experimental Section
2.1. General
2.2. Materials
2.3. Syntheses and Preparation of Materials
2.4. Analyses
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gon, M.; Tanaka, K.; Chujo, Y. Creative Synthesis of Organic–Inorganic Molecular Hybrid Materials. Bull. Chem. Soc. Jpn. 2017, 90, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Gon, M.; Sato, K.; Tanaka, K.; Chujo, Y. Controllable Intramolecular Interaction of 3D Arranged π-Conjugated Luminophores Based on a POSS Scaffold, Leading to Highly Thermostable and Emissive Materials. RSC Adv. 2016, 6, 78652–78660. [Google Scholar] [CrossRef]
- Suenaga, K.; Tanaka, K.; Chujo, Y. Heat-Resistant Mechanoluminescent chromism of the Hybrid Molecule Based on Boron Ketoiminate-Modified Octa-Substituted Polyhedral Oligomeric Silsesquioxane. Chem. Eur. J. 2017, 23, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Narikiyo, H.; Gon, M.; Tanaka, K.; Chujo, Y. Control of intramolecular excimer emission in luminophore-integrated ionic POSSs possessing flexible side-chains. Mater. Chem. Front. 2018, 2, 1449–1455. [Google Scholar] [CrossRef]
- Narikiyo, H.; Kakuta, T.; Matsuyama, H.; Gon, M.; Tanaka, K.; Chujo, Y. Development of Optical Sensor for Discriminating Isomers of Fatty Acids Based on Emissive Network Polymers Composed of Polyhedral Oligomeric Silsesquioxane. Bioorg. Med. Chem. 2017, 25, 3431–3436. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, T.; Tanaka, K.; Chujo, Y. Synthesis of Emissive Water-Soluble Network Polymers Based on Polyhedral Oligomeric Silsesquioxane and Their Application as an Optical Sensor for Discriminating the Particle Size. J. Mater. Chem. C 2015, 3, 12539–12545. [Google Scholar] [CrossRef]
- Li, Z.; Kong, J.; Wang, F.; He, C. Polyhedral oligomeric silsesquioxanes (POSSs): An important building block for organic optoelectronic materials. J. Mater. Chem. C 2017, 5, 5283–5298. [Google Scholar] [CrossRef]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef]
- Zhang, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS)nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Kausar, A. State-of-the-Art Overview on Polymer/POSS Nanocomposite. Polym. Plast. Technol. Eng. 2017, 56, 1401–1420. [Google Scholar] [CrossRef]
- Chruściel, J.J.; Leśniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 14, 67–121. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Otaigbe, J.U. Recent advances in synthesis, characterization and rheological properties of polyurethanes and POSS/polyurethane nanocomposites dispersions and films. Prog. Polym. Sci. 2009, 38, 1283–1332. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Bottino, F.A. Mono substituted octaphenyl POSSs: The effects of substituents on thermal properties and solubility. Thermochim. Acta 2017, 655, 117–123. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Liu, B.; Huang, Y.; Song, A.; Lin, Y.; Wang, M.; Li, X.; Cao, H. Gel polymer electrolyte based on polymethyl methacrylate matrixcomposited with methacrylisobutyl-polyhedral oligomericsilsesquioxane by phase inversion method. Electrochim. Acta 2018, 278, 1–12. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Bottino, P.; Chiacchio, M.A. A novel three-cages POSS molecule: synthesis and thermal behaviour. J. Therm. Anal. Calorim. 2018, 134, 1337–1344. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Bottino, F.A. Synthesis and thermal behaviour of phenyl-substituted POSSs linked by aliphatic and aromatic bridges. J. Therm. Anal. Calorim. 2018, 131, 843–851. [Google Scholar] [CrossRef]
- Maegawa, T.; Irie, Y.; Fueno, H.; Tanaka, K.; Naka, K. Synthesis and polymerization of a para-disubstituted T8-caged hexaisobutyl-POSS monomer. Chem. Lett. 2014, 43, 1532–1534. [Google Scholar] [CrossRef]
- Chujo, Y.; Tanaka, K. New polymeric materials based on element-blocks. Bull. Chem. Soc. Jpn. 2015, 88, 633–643. [Google Scholar] [CrossRef]
- Gon, M.; Tanaka, K.; Chujo, Y. Recent Progress in the Development of Advanced Element-Block Materials. Polym. J. 2018, 50, 109–126. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Advanced Functional Materials Based on Polyhedral Oligomeric Silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 1733–1746. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Unique Properties of Amphiphilic POSS and Their Applications. Polym. J. 2013, 45, 247–254. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Chemicals-Inspired Biomaterials; Developing Biomaterials Inspired by Material Science Based on POSS. Bull. Chem. Soc. Jpn. 2013, 86, 1231–1239. [Google Scholar] [CrossRef]
- Okada, H.; Tanaka, K.; Chujo, Y. Preparation of Environmentally Resistant Conductive Silica-Based Polymer Hybrids Containing Tetrathiafulvalen-Tetracyanoquinodimethane Charge-Transfer Complexes. Polym. J. 2014, 46, 800–805. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Nagai, A.; Tanaka, K.; Chujo, Y. Efficient Simultaneous Emission from RGB-Emitting Organoboron Dyes Incorporated into Organic-Inorganic Hybrids and Preparation of White Light-Emitting Materials. J. Mater. Chem. C 2013, 1, 4437–4444. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Tanaka, K.; Chujo, Y. Enhancement of Dye Dispersibility in Silica Hybrids through Local Heating Induced by the Imidazolium Group under Microwave Irradiation. Polym. J. 2014, 46, 195–199. [Google Scholar] [CrossRef]
- Okada, H.; Tanaka, K.; Chujo, Y. Regulation of Responsiveness of Phosphorescence toward Dissolved Oxygen Concentration by Modulating Polymer Contents in Organic−Inorganic Hybrid Materials. Bioorg. Med. Chem. 2014, 22, 3141–3145. [Google Scholar] [CrossRef]
- Okada, H.; Tanaka, K.; Ohashi, W.; Chujo, Y. Photo-Triggered Molecular Release Based on Auto-Degradable Polymer-Containing Organic−Inorganic Hybrids. Bioorg. Med. Chem. 2014, 22, 3435–3440. [Google Scholar] [CrossRef]
- Tanaka, K.; Adachi, S.; Chujo, Y. Structure-Property Relationship of Octa-Substituted POSS in Thermal and Mechanical Reinforcements of Conventional Polymers. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5690–5697. [Google Scholar] [CrossRef]
- Tanaka, K.; Adachi, S.; Chujo, Y. Side-Chain Effect of Octa-Substituted POSS Fillers on Refraction in Polymer Composites. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5712–5717. [Google Scholar] [CrossRef]
- Niemczyk, A.; Dziubek, K.; Sacher-Majewska, B.; Czaja, K.; Dutkiewicz, M.; Marciniec, B. Study of thermal properties of polyethylene and polypropylene nanocomposites with long alkyl chain-substituted POSS fillers. J. Therm. Anal. Calorim. 2016, 125, 1287–1299. [Google Scholar] [CrossRef] [Green Version]
- Yuasa, S.; Sato, Y.; Imoto, H.; Naka, K. Fabrication of composite films with poly(methyl methacrylate) and incompletely condensed cage-silsesquioxane fillers. J. Appl. Polym. Sci. 2018, 135, 46033. [Google Scholar] [CrossRef]
- Ueda, K.; Tanaka, K.; Chujo, Y. Remarkably High Miscibility of Octa-Substituted POSS with Commodity Conjugated Polymers and Molecular Fillers for the Improvement of Homogeneities of Polymer Matrices. Polym. J. 2016, 48, 1133–1139. [Google Scholar] [CrossRef]
- Tanaka, K.; Yamane, H.; Mitamura, K.; Watase, S.; Matsukawa, K.; Chujo, Y. Transformation of Sulfur to Organic−Inorganic Hybrids Employed by POSS Networks and Their Application for the Modulation of Refractive Indices. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2588–2595. [Google Scholar] [CrossRef]
- Ueda, K.; Tanaka, K.; Chujo, Y. Synthesis of POSS Derivatives Having Dual Types of Alkyl Substituents via in situ Sol−Gel Reactions and Their Application as a Molecular Filler for Low-Refractive and Highly-Durable Materials. Bull. Chem. Soc. Jpn. 2017, 90, 205–209. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Tanaka, K.; Chujo, Y. Rational Design of POSS Fillers for Simultaneous Improvements of Thermomechanical Properties and Lowering Refractive Indices of Polymer Films. J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 3583–3589. [Google Scholar] [CrossRef]
- Mont, F.W.; Schubert, E.F. High-refractive-index TiO2-nanoparticle-loaded encapsulants for light-emitting diodes. J. Appl. Phys. 2008, 103, 083120. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, H.S.; Kim, E.K.; Baek, K.-Y.; Choi, D.-H.; Hwang, S.S. Synthesis and Characterization of Fluoro-co-Phenyl Silsesquioxane (FCPSQ) for Low Dielectric Constant Materials. Mol. Cryst. Liq. Cryst. 2010, 520, 231–238. [Google Scholar] [CrossRef]
- Ro, H.W.; Kim, K.J.; Theato, P.; Gidley, D.W.; Yoon, D.Y. Novel inorganic-organic hybrid block copolymers as pore generators for nanoporous ultralow-dielectric-constant films. Macromolecules 2005, 38, 1031–1034. [Google Scholar] [CrossRef]
- Hedrick, J.L.; Miller, R.D.; Hawker, C.J.; Carter, K.R.; Volksen, W.; Yoon, D.Y.; Trollsås, M. Templating nanoporosity in thin-film dielectric insulators. Adv. Mater. 1998, 10, 1049–1053. [Google Scholar] [CrossRef]
- Tanaka, K.; Kozuka, H.; Ueda, K.; Jeon, J.-H.; Chujo, Y. POSS-Based Molecular Fillers for Simultaneously Enhancing Thermal and Viscoelasticity of Poly(methyl methacrylate) Films. Mater. Lett. 2017, 203, 62–67. [Google Scholar] [CrossRef]
- Groh, W.; Zimmermann, A. What Is the Lowest Refractive Index of an Organic Polymer? Macromolecules 1991, 24, 6660–6663. [Google Scholar] [CrossRef]
- Tanio, N.; Irie, M. Estimate of Light Scattering Loss of Amorphous Polymer Glass from Its Molecular Structure. Jpn. J. Appl. Phys. Part I 1997, 36, 743–748. [Google Scholar] [CrossRef]
- Evert, A.; James, A.; Hawkins, T.; Foy, P.; Stolen, R.; Dragic, P.; Dong, L.; Rice, R.; Ballato, J. Longitudinally-graded optical fibers. Opt. Express 2012, 20, 17393–17401. [Google Scholar] [CrossRef]
- Hutsel, M.R.; Gaylord, T.K. Concurrent three-dimensional characterization of the refractive-index and residual-stress distributions in optical fibers. Appl. Opt. 2012, 51, 5442–5452. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Inaba, A.; Brown, J.E.; Hatada, K.; Kitayama, T.; Masuda, E. Effects of weak linkages on the thermal and oxidative degradation of poly(methyl methacrylates). Macromolecules 1986, 19, 2160–2168. [Google Scholar] [CrossRef]



| POSS filler | Transmittance (%) b | ∆n c | Kp2 | Td20 (°C) d | ΔTd20 (°C) | Tg (°C) | E’ (MPa) e |
|---|---|---|---|---|---|---|---|
| none | 92 | − | − | 215 | − | 60.6 | 1780 |
| F+MMA | 87 | −0.0197 | 0.093 | 268 | +53 | 63.8 | 3060 |
| F+CP | 85 | −0.0147 | 0.180 | 225 | +10 | 61.8 | 3050 |
| F+C18 | 45 | −f | − f | 272 | +57 | 58.6 | 2630 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, K.; Tanaka, K.; Chujo, Y. Fluoroalkyl POSS with Dual Functional Groups as a Molecular Filler for Lowering Refractive Indices and Improving Thermomechanical Properties of PMMA. Polymers 2018, 10, 1332. https://doi.org/10.3390/polym10121332
Ueda K, Tanaka K, Chujo Y. Fluoroalkyl POSS with Dual Functional Groups as a Molecular Filler for Lowering Refractive Indices and Improving Thermomechanical Properties of PMMA. Polymers. 2018; 10(12):1332. https://doi.org/10.3390/polym10121332
Chicago/Turabian StyleUeda, Kazunari, Kazuo Tanaka, and Yoshiki Chujo. 2018. "Fluoroalkyl POSS with Dual Functional Groups as a Molecular Filler for Lowering Refractive Indices and Improving Thermomechanical Properties of PMMA" Polymers 10, no. 12: 1332. https://doi.org/10.3390/polym10121332