Preparation and Properties of SBS-g-GOs-Modified Asphalt Based on a Thiol-ene Click Reaction in a Bituminous Environment
Abstract
:1. Introduction
2. Preparation of SBS-g-GOs-Modified Asphalt
2.1. Materials
2.2. Preparation Method
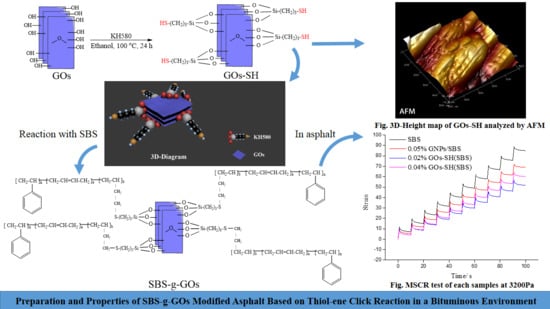
2.2.1. Preparation of GOs-SH
2.2.2. Preparation of SBS-g-GOs-modified asphalt
2.3. Performance and Characterization Analysis
3. Results and Discussion
3.1. Three Indicators
3.2. DSR Test
3.2.1. Variation of G′ with GOs-SH Content
3.2.2. Effect of GOs-SH Content on tanδ and G′′
3.2.3. Determination of the High-Temperature Asphalt Grade (PG Grade)
3.3. MSCR Test
3.4. Stability of SBS-g-GOs in Asphalt
3.5. Microscopic Analysis
3.5.1. Analysis of SBS-g-GOs in Asphalt via FM
3.5.2. Microscopic Representation of GOs-SH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, P.; Dong, Z.J.; Tan, Y.Q.; Liu, Z.Y. Effect of multi-walled carbon nanotubes on the performance of styrene–butadiene–styrene copolymer modified asphalt. Mater. Struct. 2017, 50, 17. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Li, M.; Li, L. Evaluation of pavement responses and performance with thermal modified asphalt mixture. Mater. Des. 2016, 111, 88–97. [Google Scholar] [CrossRef]
- Baek, J.; Sang, Y.L.; Lee, H.J. Comparative evaluation of wma additives effects on conventional and polymer modified asphalt pavements. KSCE J. Civ. Eng. 2018, 22, 2099–2108. [Google Scholar] [CrossRef]
- Liang, M.; Xin, X.; Fan, W.; Ren, S.; Shi, J.; Luo, H. Thermo-stability and aging performance of modified asphalt with crumb rubber activated by microwave and TOR. Mater. Des. 2017, 127, 84–96. [Google Scholar] [CrossRef]
- Santagata, E.; Baglieri, O.; Dalmazzo, D.; Tsantilis, L. Evaluation of the anti-rutting potential of polymer-modified binders by means of creep-recovery shear tests. Mater. Struct. 2013, 46, 1673–1682. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Zhao, W.; Zhang, Y.; Wei, J.; Fan, W.; Yu, Y.; Wang, Z. Influence of SBS and asphalt on SBS dispersion and the performance of modified asphalt. Constr. Build. Mater. 2014, 62, 1–7. [Google Scholar] [CrossRef]
- Hou, D.; Han, M.; Muhammad, Y.; Liu, Y.; Zhang, F.; Yin, Y.; Duan, S.; Li, J. Performance evaluation of modified asphalt based trackless tack coat materials. Constr. Build. Mater. 2018, 165, 385–394. [Google Scholar] [CrossRef]
- Goli, A.; Ziari, H.; Amini, A. Influence of carbon nanotubes on performance properties and storage stability of SBS modified asphalt binders. J. Mater. Civ. Eng. 2017, 29, 04017070. [Google Scholar] [CrossRef]
- Pang, J.; Du, S.; Chang, R.; Cui, D. Rheological properties of SBS-modified asphalt in the presence of dithiodimorpholine and tetraethyl thiuram disulfide. Polym. Compos. 2016, 37, 943–948. [Google Scholar] [CrossRef]
- Sengul, C.E.; Oruc, S.; Iskender, E.; Aksoy, A. Evaluation of SBS modified stone mastic asphalt pavement performance. Constr. Build. Mater. 2013, 41, 777–783. [Google Scholar] [CrossRef]
- Wu, G.; Zeng, S.; Ou, E.; Yu, P.; Lu, Y.; Xu, W. Photoinitiator grafted styrene–butadiene–styrene triblock copolymer. Mater. Sci. Eng. C 2010, 30, 1030–1037. [Google Scholar] [CrossRef]
- Cong, P.; Chen, S.; Chen, H. Preparation and properties of bitumen modified with the maleic anhydride grafted styrene-butadiene-styrene triblock copolymer. Polym. Eng. Sci. 2011, 51, 1273–1279. [Google Scholar] [CrossRef]
- Kennedy, J.E.; Lyons, J.G.; Geever, L.M.; Higginbotham, C.L. Synthesis and characterisation of styrene butadiene styrene-g-acrylic acid for potential use in biomedical applications. Mater. Sci. Eng. C 2009, 29, 1655–1661. [Google Scholar] [CrossRef]
- Mitov, Z.; Velichkova, R. Modification of styrene-isoprene block copolymers—3. Addition of maleic anhydride—Mechanism. Eur. Polym. J. 1993, 29, 597–601. [Google Scholar] [CrossRef]
- Hsiue, G.H.; Huang, W.K.; Hou, W.H. Dynamic mechanical and dielectric properties of epoxidized SBS triblock copolymer. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 4119–4128. [Google Scholar] [CrossRef]
- Zhang, A.; Li, C. Chemical initiation mechanism of maleic anhydride grafted onto styrene–butadiene–styrene block copolymer. Eur. Polym. J. 2003, 39, 1291–1295. [Google Scholar] [CrossRef]
- Decker, C.; Nguyen Thi Viet, T. High-speed photocrosslinking of thermoplastic styrene–butadiene elastomers. J. Appl. Polym. Sci. 2015, 77, 1902–1912. [Google Scholar] [CrossRef]
- Schapman, F.; Couvercelle, J.P.; Bunel, C. Low molar mass polybutadiene made crosslinkable by silane moities introduced via addition of thiol to double bond: 4. Crosslinking study. Polymer 2000, 41, 17–25. [Google Scholar] [CrossRef]
- Lotti, L.; Coiai, S.; Ciardelli, F.; Galimberti, M.; Passaglia, E. Thiol-ene radical addition of l-cysteine derivatives to low molecular weight polybutadiene. Macromol. Chem. Phys. 2010, 210, 1471–1483. [Google Scholar] [CrossRef]
- Li, X.K.; Chen, G.S.; Duan, M.W.; Yang, W.C.; Tang, S.C.; Cao, Y.D.; Luo, Y.; Li, X.K.; Chen, G.S.; Duan, M.W. Branched hydroxyl modification of SBS using Thiol-ENE reaction and its subsequent application in modified asphalt. Ind. Eng. Chem. Res. 2017, 56, 10354–10365. [Google Scholar] [CrossRef]
- Xi, W.; Scott, T.F.; Kloxin, C.J.; Bowman, C.N. Click chemistry in materials science. Adv. Funct. Mater. 2014, 24, 2572–2590. [Google Scholar] [CrossRef]
- Guo, J.; Xie, Z.; Tran, R.T.; Xie, D.; Yang, J. Click chemistry plays a dual role in biodegradable polymer design. Adv. Mater. 2014, 26, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Lodge, T.P. A virtual issue of macromolecules: “Click chemistry in macromolecular science”. Macromolecules 2015, 42, 3827–3829. [Google Scholar] [CrossRef]
- Logan, A.; Pell, V.R.; Shaffer, K.J.; Evans, C.; Stanley, N.J.; Robb, E.L.; Prime, T.A.; Chouchani, E.T.; Cochemé, H.M.; Fearnley, I.M. Assessing the mitochondrial membrane potential in cells and in vivo using targeted click chemistry and mass spectrometry. Cell MeTable 2016, 23, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Döhler, D.; Michael, P.; Binder, W.H. CuAAC-based click chemistry in self-healing polymers. Acc. Chem. Res. 2017, 50, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Bian, T.; Wang, C.; Lu, Z.; Xie, R.; Yang, Q.Z.; Wu, L.Z.; Tung, C.H.; Liu, Z.; Yin, Y.; Zhang, T. Nanocrystals: A versatile ‘click chemistry’ route to size-restricted, robust, and functionalizable hydrophilic nanocrystals (small 14/2015). Small 2015, 1613. [Google Scholar] [CrossRef]
- Elchinger, P.H.; Faugeras, P.A.; Boens, B.; Brouillette, F.O.; Montplaisir, D.; Zerrouki, R.; Lucas, R. Polysaccharides: The “click” chemistry impact. Polymers 2011, 3, 1607–1651. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Rabek, J.F. Radiation Curing in Polymer Science and Technology; Elsevier Applied Science: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Hoyle, C.E. Thiol–enes: Chemistry of the past with promise for the future. J. Polym. Sci. Part A Polym. Chem. 2010, 42, 5301–5338. [Google Scholar] [CrossRef]
- Antoni, P.; Robb, M.J.; Campos, L.; Montanez, M.; Hult, A.; Malmström, E.; Malkoch, M.; Hawker, C.J. Pushing the limits for thiol−ene and CuAAC reactions: Synthesis of a 6th generation dendrimer in a single day. Macromolecules 2010, 43, 6625–6631. [Google Scholar] [CrossRef]
- Montañez, M.I.; Campos, L.M.; Antoni, P.; Hed, Y.; Walter, M.V.; Krull, B.T.; Khan, A.; Hult, A.; Hawker, C.J.; Malkoch, M. Accelerated growth of dendrimers via thiol−ene and esterification reactions. Macromolecules 2010, 43, 6004–6013. [Google Scholar] [CrossRef]
- Lim, Y.; Heo, G.S.; Rezenom, Y.H.; Pollack, S.; Raymond, J.E.; Elsabahy, M.; Wooley, K.L. Development of a vinyl ether-functionalized polyphosphoesteras a template for multiple postpolymerization conjugation chemistriesand study of core degradable polymeric nanoparticles. Macromolecules 2014, 47, 4634–4644. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bartels, J.W.; Li, Z.; Zhang, K.; Cheng, C.; Wooley, K.L. Synthesis and solution-state assembly or bulk state thiol-ene crosslinking of pyrrolidinone- and alkene-functionalized amphiphilic block fluorocopolymers: From functional nanoparticles to anti-fouling coatings. Aust. J. Chem. 2010, 63, 1159–1163. [Google Scholar] [CrossRef]
- Ma, J.; Cheng, C.; Wooley, K.L. The power of raft for creating polymers having imbedded side-chain functionalities: Norbornenyl-functionalized polymers and their transformations via romp and thiol-ene reactions. Aust. J. Chem. 2009, 62, 1507–1519. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Q.; Li, Z.; Fan, G.; Xiong, D.; Su, Y.; Zhang, J.; Zhang, D. Enhanced mechanical properties of graphene (reduced graphene oxide)/aluminum composites with a bioinspired nanolaminated structure. Nano Lett. 2015, 15, 8077–8083. [Google Scholar] [CrossRef] [PubMed]
- Guardia, L.; Villar-Rodil, S.; Paredes, J.I.; Rozada, R.; Martínez-Alonso, A.; Tascón, J. UV light exposure of aqueous graphene oxide suspensions to promote their direct reduction, formation of graphene–metal nanoparticle hybrids and dye degradation. Carbon 2012, 50, 1014–1024. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Li, C.; Qin, C.; Ye, M. Synthesis of amphiphilic graphene nanoplatelets. Small 2010, 5, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xiao, F.; Amirkhanian, S.; You, Z.; Huang, J. Developments of nano materials and technologies on asphalt materials—A review. Constr. Build. Mater. 2017, 143, 633–648. [Google Scholar] [CrossRef]
- Gao, C.M.; Han, S.; Chen, S.; Li, H. Research on basalt fiber asphalt concrete’s low temperature performance. Appl. Mech. Mater. 2014, 505–506, 35–38. [Google Scholar] [CrossRef]
- Le, J.; Marasteanu, M.; Turos, M. Graphene Nanoplatelet (GNP) Reinforced Asphalt Mixtures: A Novel Multifunctional Pavement Material; Transportation Research Board: Washington, WA, USA, 2016; Volume 173, pp. 1–39. [Google Scholar]
- Han, M.; Li, J.; Muhamma, Y.; Hou, D.; Zhang, F.; Yin, Y.; Duan, S. Effect of polystyrene grafted graphene nanoplatelets on the physical and chemical properties of asphalt binder. Constr. Build. Mater. 2018, 174, 108–119. [Google Scholar] [CrossRef]
- Han, M.; Li, J.; Muhammad, Y.; Yin, Y.; Yang, J.; Yang, S.; Duan, S. Studies on the secondary modification of SBS modified asphalt by the application of octadecyl amine grafted graphene nanoplatelets as modifier. Diam. Relat. Mater. 2018, 89, 140–150. [Google Scholar] [CrossRef]
- Li, J.; Han, M.; Muhammad, Y.; Liu, Y.; Yang, S.; Duan, S.; Huang, W.; Zhao, Z. Comparative analysis, road performance and mechanism of modification of polystyrene graphene nanoplatelets (PS-GNPS) and octadecyl amine graphene nanoplatelets (ODA-GNPS) modified SBS incorporated asphalt binders. Constr. Build. Mater. 2018, 193, 501–517. [Google Scholar] [CrossRef]
- Airey, G.D. Styrene butadiene styrene polymer modification of road bitumens. J. Mater. Sci. 2004, 39, 951–959. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; Yaseen, M.; Han, M.; Yin, Y.; Yang, S. Preparation methods and performance of modified asphalt using rubber–plastic alloy and its compounds. J. Mater. Civ. Eng. 2018, 30, 04018163. [Google Scholar] [CrossRef]
- Zhang, F.; Muhammad, Y.; Liu, Y.; Han, M.; Yin, Y.; Hou, D.; Li, J. Measurement of water resistance of asphalt based on surface free energy analysis using stripping work between asphalt-aggregate system. Constr. Build. Mater. 2018, 176, 422–431. [Google Scholar] [CrossRef]
- Ministry of Transport of the People’s Republic of China. Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering: JTG E20-2011; China Communications Press: Beijing, China, 2011.
- Jahanbakhsh, H.; Karimi, M.M.; Nejad, F.M.; Jahangiri, B. Viscoelastic-based approach to evaluate low temperature performance of asphalt binders. Constr. Build. Mater. 2016, 128, 384–398. [Google Scholar] [CrossRef]
- Yang, X.; You, Z. High temperature performance evaluation of bio-oil modified asphalt binders using the DSR and MSCR tests. Constr. Build. Mater. 2015, 76, 380–387. [Google Scholar] [CrossRef]
- Huang, W.; Tang, N. Characterizing SBS modified asphalt with sulfur using multiple stress creep recovery test. Constr. Build. Mater. 2015, 93, 514–521. [Google Scholar] [CrossRef]
- Shaheen, M.; Al-Mayah, A.; Tighe, S. Optimization of hot-mix asphalt surface course mix design for fatigue resistance: High-friction aggregate and PG plus. J. Mater. Civ. Eng. 2016, 28, 04015172. [Google Scholar] [CrossRef]
- Norouzi, A.; Kim, Y.R.; Kim, S.S.; Yang, J. Effect of reclaimed asphalt pavement content and binder grade on fatigue-resisting performance of asphalt mixtures in Georgia. J. Mater. Civ. Eng. 2017, 29, 04017115. [Google Scholar] [CrossRef]
- Wen, T.; Wu, X.; Tan, X.; Wang, X.; Xu, A. One-pot synthesis of water-swellable mg-al layered double hydroxides and graphene oxide nanocomposites for efficient removal of As(V) from aqueous solutions. ACS Appl. Mater. Interfaces 2013, 5, 3304–3311. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Ma, Z.; Qiu, Y.; Yang, H.; Huang, Y.; Liu, J.; Lu, Y.; Zhang, C.; Hu, P. Effective synergistic effect of dipeptide-polyoxometalate-graphene oxide ternary hybrid materials on peroxidase-like mimics with enhanced performance. ACS Appl. Mater. Interfaces 2015, 7, 22036–22045. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, G.; Yang, W.; Duan, M.; Tang, S.; Cao, Y.; Mai, Y.; Luo, Y. Carboxyl branched-functionalized modification of SBS and application to modified asphalt. Eng. Plast. Appl. 2017, 45, 121–125. [Google Scholar]
- Li, S.M.; Wang, Y.S.; Hsiao, S.T.; Liao, W.H.; Lin, C.W.; Yang, S.Y.; Tien, H.W.; Ma, C.C.M.; Hu, C.C. Fabrication of a silver nanowire-reduced graphene oxide-based electrochemical biosensor and its enhanced sensitivity in the simultaneous determination of ascorbic acid, dopamine, and uric acid. J. Mater. Chem. C 2015, 3, 9444–9453. [Google Scholar] [CrossRef]
- Zhu, C.; Zhai, J.; Wen, D.; Dong, S. Graphene oxide/polypyrrole nanocomposites: One-step electrochemical doping, coating and synergistic effect for energy storage. J. Mater. Chem. 2012, 22, 6300–6306. [Google Scholar] [CrossRef]
- Ren, L.; Wang, X.; Guo, S.; Liu, T. Functionalization of thermally reduced graphene by in situ atom transfer radical polymerization. J. Nanopart. Res. 2011, 13, 6389–6396. [Google Scholar] [CrossRef]
- Murdock, A.T.; Koos, A.; Britton, T.B.; Houben, L.; Batten, T.; Zhang, T.; Wilkinson, A.J.; Duninborkowski, R.E.; Lekka, C.E.; Grobert, N. Controlling the orientation, edge geometry, and thickness of chemical vapor deposition graphene. ACS Nano 2013, 7, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.D.; Taejoon Han, A.; Beebe, T.P. Adsorption of 11-mercaptoundecanoic acid on ni(111) and its interaction with probe molecules. Langmuir 1997, 13, 3397–3403. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: Scienta ESCA300 Database; Wiley: Hoboken, NJ, USA, 1992; p. A25. [Google Scholar]
- Duan, W.; Li, M.; Sun, H.; Cheng, J.; Zhang, J. Preparation and characterization of thiol-ene/epoxy photo-thermal double curing system. Bonding 2017, 38, 16–22. [Google Scholar]
- Andreu, N.; Flahaut, D.; Dedryvere, R.; Minvielle, M.; Martinez, H.; Gonbeau, D. XPS investigation of surface reactivity of electrode materials: Effect of the transition metal. ACS Appl. Mater. Interfaces 2015, 7, 6629–6636. [Google Scholar] [CrossRef] [PubMed]
- Hai, G.H.; Ying, H.C.; Jing, Y.H.; Hua Tang, H.; Guo, Q.X. Formation of a tetra-σ-bonded intermediate in acetylethyne binding on si(100)-2 × 1. Langmuir 2005, 21, 3384–3388. [Google Scholar]
- Luckas, N.; Gotterbarm, K.; Streber, R.; Lorenz, M.P.; Höfert, O.; Viñes, F.; Papp, C.; Görling, A.; Steinrück, H.P. Adsorption and reaction of SO2 on clean and oxygen precovered Pd(100)-a combined HR-XPS and DF study. Phys. Chem. Chem. Phys. 2011, 13, 16227–16235. [Google Scholar] [CrossRef] [PubMed]
- Dalili, N.; He, A.; Liu, Q.; Ivey, D.G. Erratum to: A cryo-xps study of triammonium citrate-kaucl4-na2so3 electroplating solutions for pb-free solder packaging. J. Electron. Mater. 2010, 39, 1554–1561. [Google Scholar] [CrossRef]
- Yang, X.; Liu, K. Study on mechanism of sulphur modified asphalt. J. Shijiazhuang Railw. Inst. 2008, 21, 43–48. [Google Scholar]
- Yang, X.; Xiong, S.; Jiao, S.; Liu, K. Investigation on the properties of sulphur modified asphalt mixture and it’s modifying mechanism. J. Hunan Univ. Sci. Technol. 2009, 24, 61–68. [Google Scholar]
- Iii, S.B.C.; Mohammad, L.N.; Elseifi, M.A. Laboratory performance characteristics of sulfur-modified warm-mix asphalt. J. Mater. Civ. Eng. 2011, 23, 1338–1345. [Google Scholar]






















| θ/°C | SBS | 0.05% GNPs/SBS | 0.02% GOs-SH (SBS) | 0.04% GOs-SH (SBS) | 0.06% GOs-SH (SBS) |
|---|---|---|---|---|---|
| 46 | 49.55 | 54.86 | 70.04 | 62.34 | 51.33 |
| 52 | 26.21 | 28.52 | 34.43 | 29.86 | 26.21 |
| 58 | 15.16 | 16.01 | 18.07 | 15.00 | 13.96 |
| 64 | 9.35 | 8.75 | 10.14 | 8.17 | 7.52 |
| 70 | 5.79 | 5.06 | 5.96 | 4.66 | 4.18 |
| 76 | 3.66 | 3.50 | 3.59 | 2.73 | 2.47 |
| 82 | 2.41 | 2.24 | 2.19 | 1.65 | 1.60 |
| RTFOT | |||||
| 76 | - | - | - | - | 2.88 |
| 82 | 2.74 | 2.46 | 2.41 | 2.58 | 1.97 |
| PG | 82 | 82 | 82 | 82 | 76 |
| Type/Unit | SBS | 0.05% GNPs/SBS | 0.02% GOs-SH (SBS) | 0.04% GOs-SH (SBS) |
|---|---|---|---|---|
| R0.1 | 0.675 | 0.686 | 0.786 | 0.745 |
| R3.2 | 0.341 | 0.368 | 0.494 | 0.417 |
| Jnr0.1/ kPa−1 | 0.856 | 0.826 | 0.553 | 0.660 |
| Jnr3.2/ kPa−1 | 2.655 | 2.158 | 1.618 | 1.882 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Han, M.; Muhammad, Y.; Liu, Y.; Su, Z.; Yang, J.; Yang, S.; Duan, S. Preparation and Properties of SBS-g-GOs-Modified Asphalt Based on a Thiol-ene Click Reaction in a Bituminous Environment. Polymers 2018, 10, 1264. https://doi.org/10.3390/polym10111264
Li J, Han M, Muhammad Y, Liu Y, Su Z, Yang J, Yang S, Duan S. Preparation and Properties of SBS-g-GOs-Modified Asphalt Based on a Thiol-ene Click Reaction in a Bituminous Environment. Polymers. 2018; 10(11):1264. https://doi.org/10.3390/polym10111264
Chicago/Turabian StyleLi, Jing, Meizhao Han, Yaseen Muhammad, Yu Liu, Zhibin Su, Jing Yang, Song Yang, and Shaochan Duan. 2018. "Preparation and Properties of SBS-g-GOs-Modified Asphalt Based on a Thiol-ene Click Reaction in a Bituminous Environment" Polymers 10, no. 11: 1264. https://doi.org/10.3390/polym10111264