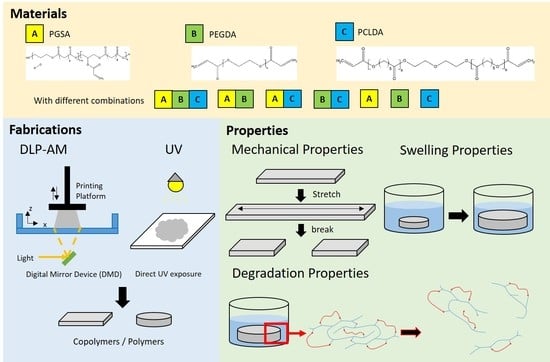
1. Introduction
Acrylated polymers are highly popular in the glue [
1,
2] and coating [
3,
4,
5] industry. They are well known for the crosslinkability between the double bonds and their fast reaction rates. The radical polymerization that acrylated polymers go through generally require an initiation process, which may be triggered by heat, catalyst, or light [
6,
7]. The rapid reaction and photocurable properties of some of the acrylated polymers have also led to their applications toward 3D printing [
8,
9].
Stereolithography (SLA) and digital light processing additive manufacturing (DLP-AM) are two of the 3D printing methods involving radical polymerizations triggered via light. Both of the fabrication methods are considered highly precise and efficient compared to fused deposition modeling (FDM) [
10,
11]. Unlike FDM, which relies on the extrusion of molten thermoplastics, SLA and DLP-AM initiates the crosslinking between acrylated polymer chains via the exposure to light at various wavelengths. SLA is known for the use of single laser beam (UV) as the light source, while DLP-AM employs the digital mirror device (DMD) to control the exposure of light in an array simultaneously [
12]. SLA is known to be more time-consuming, but capable of high resolutions as low as 2 μm [
11]. Meanwhile, DLP-AM systems are capable of rapid prototyping and are cheaper than typical SLA systems due to the wider options in light sources [
13]. Both additive manufacturing methods have gained lots of attention and opened up new markets over the past decades. However, the brittle nature and toxicity of the acrylated polymers limited the applications of the two systems toward the biomedical field, especially in tissue engineering. More and more attention has been focused on the development of biocompatible or biodegradable acrylates over the years [
14,
15,
16].
In the search for acrylated polymers that may be applied in biomedicine, many studies have been dedicated to the conversion of existing biocompatible/biodegradable polymers—such as poly(lactic-
co-glycolic acid) (PLGA), polycaprolactone (PCL), and polyethylene glycol (PEG)—into photocurable polymer by introducing acrylate groups to the polymers. In the work of Ge, et al., PLGA has been applied in the fabrication of 3D printed scaffolds and cultured with human fetal osteoblasts [
17] and osteosarcoma cells [
18] for bone tissue regeneration. PEG has also been converted into polyethylene glycol diacrylate (PEGDA), which are readily purchasable, for various applications, including scaffolds for cell culture with bone marrow stromal cells [
19] and human mesenchymal stem cells [
20], and scaffolds for aortic valve [
21]. Polycaprolactone diacrylate (PCLDA) have also been synthesized and printed via LCD-projected maskless additive manufacturing system by Cheng, et al. [
22]. The emergence of the biodegradable, photocurable polymers have brought forth new options toward the fabrication of biomedical devices and scaffolds. However, photocurable polymeric materials are often criticized for their high Young’s modulus and their reduced degradation rate, limiting their applications to bone tissue engineering, and less compatible for soft tissue engineering [
23,
24]. Meanwhile, gelatin methacryloyl (GelMA) have been introduced as a photocurable hydrogel for tissue engineering, and have been shown 3D printable via proposed projection stereolithography [
25]. Yet the low Young’s modulus and fast degradation also limits the applications of GelMA to vascularization and cell culture [
26].
Poly(glycerol sebacate) (PGS) was first developed in MIT in 2002, known as the biorubber [
27]. Its high elasticity and low Young’s modulus along with the bioabsorbability made it a good choice of material for soft tissue engineering, such as heart [
28] and arterial [
29]. In 2007, photocurable, biodegradable polymer poly(glycerol sebacate) acrylate (PGSA) is introduced by adding acrylate groups to PGS by Nijst, et al. [
30]. Compared to PGS, which is an elastomer that typically requires a secondary cure ranging between 8–48 h [
27,
31,
32]. PGSA is a photocurable elastomer which may be cured via UV curing at room temperature for 10 min [
30]. In this work, we propose the co-polymerization of PGSA and PEGDA or PCLDA at various degree of acrylation and ratio to provide a wide range of selection of biodegradable, photocurable polymers for soft tissue engineering. Through the combination of two or three of the abovementioned prepolymers, the mechanical and degradation properties can be easily modified. If combined with suitable blending, delivery, and curing setups, scaffolds with varying mechanical properties maybe fabricated through 3D printing.
2. Materials and Methods
All materials were purchased from Sigma-Aldrich (St. Louis, MO, USA), and used as received unless otherwise specified.
2.1. Synthesis of PGSA Prepolymer
PGSA prepolymer was synthesized according to previously published methods [
27,
30]. An equimolar mixture of glycerol and sebacic acid was added into a two-neck round-bottom flask and melted at 130 °C under nitrogen for 2 h. PGS prepolymer was formed via polycondensation at 130 °C under low pressure for 24 h and cooled to room temperature for further usage. PGS acrylation was performed as follows: 30 g of PGS prepolymer, 30 mg of 4-(dimethylamino)pyridine (DMAP) (Alfa Aesar, Ward Hill, MA, USA), and 300 mL of dichlormethane (DCM) (Echo Chemical Co., Ltd., Miaoli, Taiwan) were mixed in a two-neck round-bottom flask under nitrogen for 10 min before the reaction was cooled to 0 °C. Then triethylamine (125 mol% of acryloyl chloride amount) (Alfa Aesar, Ward Hill, MA, USA) was added, followed by slow addition of acryloyl chloride (60, 30, and 15 mol % of hydroxyl groups on PGS prepolymer for synthesis of PGSA30, 15 and 7 respectively). (Merck & Co., Kenilworth, NJ, USA), and reacted at room temperature for 24 h. The resulting mixture was rotary-evaporated, dissolved in ethyl acetate (EA) (Echo Chemical Co., Ltd., Miaoli, Taiwan) and filtered repeatedly. After mixing with 5 × 10
−6 M hydrochloric acid in a separating funnel and settled for 24 h, the prepolymer solution was pipetted out and EA was removed to obtain the PGSA prepolymer. All PGSA prepolymers are characterized via VNMRS-700 NMR spectrometer (Varian, Palo Alto, CA, USA) for the degree of acrylation. The calculation and NMR spectra are shown in
Figure S1 in the Supplemental Material. The calculated degree of acrylation in PGSA (DA = 30%) is abbreviated as PGSA30 throughout this work. Similarly, PGSA (DA = 15%) is PGSA 15 and PGSA (DA = 7%) is PGSA 7.
2.2. PCLDA Prepolymer Synthesis
Polycaprolactone diacrylate (PCLDA) was synthesized according to the work of Kweon, et al. [
33] in the following steps. 4 g (0.002 mol) of polycaprolactone diol (
Mn 2000) and 40 mL of toluene (Alfa Aesar, Ward Hill, MA, USA) were mixed in a two-neck round-bottom flask under nitrogen. After fully dissolved, nitrogen was stopped and 0.63 mL (0.0045 mol) of triethylamine and 0.37 mL (0.0045 mol) of acryloyl chloride were added, respectively. The reaction was performed at 80 °C for 3 h before cooled down to room temperature. Then triethylamine hydrochloride was removed via filtration and 400 mL of n-hexane was added. PCLDA prepolymer precipitation was collected and dried at 50 °C for 24 h to remove the solvent. The NMR spectrum is of PCLDA synthesized is shown in
Figure S2 in the Supplemental Material.
2.3. Prepolymer Blending
PGSA, PCLDA, and PEGDA (Mn 700) prepolymers were mixed with different weight percent ratios and stirred for 30 min to obtain fully mixed materials. 3 wt % of 2,4,6-trimethyl benzoyl diphenyl phosphine oxide (TPO) was added to the mixtures as photoinitiator to accelerate the photopolymerization reaction.
2.4. Polymer Rheological Properties
The rheometer MCR 302 (Anton Paar, Graz, Austria) was used to measure the viscosity of prepolymer mixture. The analysis was performed in parallel plate geometry (
d = 25 mm; gap = 0.2 mm) at shear rate ranging from 0.1 to 1000 s
−1. The measuring temperature for PCLDA prepolymer was set at 40 °C [
34], while others were set at 25 °C.
2.5. DLP-AM Printing of Polymer Film/Mechanical Tester
Digital light processing system was assembled by the Cheng lab at National Taiwan University of Science and Technology, and printing protocol was modified based on previous published methods [
22,
35]. Briefly, STL files created by SOLIDWORKS
® 2017 were processed by computer to create patterns for each layer. Prepolymer mixtures were loaded onto the loading platform where the projector (Acer, New Taipei, Taiwan) projected patterns to cure the material. After one layer was cured, the probe moves upward in
z-axis for next-layer exposure. The structure was printed layer-by-layer and removed from the probe after printing. Printed parts were washed with 95% ethanol and dried at room temperature for further tests.
2.6. Mechanical Property
Mechanical testers (30 × 3 × 0.5 mm
3) (
n = 5), prepared by DLP-AM and UV-curing respectively, were tested on an TA-ElectroForce
® 3200 Series III system (Thermal Analysis, New Castle, DE, USA) and a 225 N load cell. Specimens were extended at a rate of 0.08 mm/min until break. Ultimate tensile strength and elongation at break data were collected, and Young’s modulus (MPa) was calculated from the slope of the first 10% of the stress–strain curve. A detailed analyses of the thermal properties of the prepolymers and copolymers are shown in
Figure S3 and Table S1 in the Supplemental Material.
2.7. Degradation Property
Degradation samples (diameter = 1.5 cm; thickness = 1 mm) (
n ≥ 3) were prepared by DLP-AM and soaked subsequently into ethanol solutions with the following concentrations for 12 h each: 95%, 75%, 50%, 25%, and 0% (RO water). Samples were then dried at 50 °C for seven days and weighed before tests. Enzyme degradation was carried out according to Hsieh, et al. [
36] and Lin, et al. [
37]. In short, lipase from porcine pancreas Type II was dissolved in phosphate buffer solution (20 units/mL) and filtrated before use. Degradation samples and 4 mL of enzyme solution were placed in 12-well cell culture plates. Then the enzyme degradation was carried out at 37 °C with enzyme solutions changed every two days. Samples were collected, washed with distilled water, dried at 50 °C for seven days and weighed again to determine the mass loss.
2.8. Swelling Ratio
After the degraded samples were washed, dried and weighed. The samples were soaked in PBS solution at 37 °C for 24 h and weighed again to determine the wet weight. The swelling ratio was calculated from the equation [
38]
2.9. NaOH Degradation
NaOH degradation was modified from the protocols of Lam, et al. [
39] with modifications to the sample sizes and NaOH concentration. Degradation samples (diameter = 1 cm; thickness = 0.5 mm) (
n ≥ 3) were prepared by DLP-AM, and soaked subsequently into ethanol solutions with the following concentrations for 12 h each: 95%, 75%, 50%, 25%, and 0% (RO water). Samples were then dried at 50 °C for seven days to determine the dry weight. Two groups of dried samples were soaked into 0.1 M NaOH solution at 37 °C for 24 and 48 h respectively. After degradation, samples were washed with RO water, dried at 50 °C for seven days and weighed again to determine the mass loss.
2.10. UV Film Formation
Prepolymer blends were pipetted into metal molds and covered with microscope slides (1 mm thick) on each side. Then the assembly was exposed to ultraviolet light (UV curing box, Hg lamp with wavelength 365 nm, ~12 mW/cm2) for 30 s per side. Polymer films (70 mm × 20 mm × 0.5 mm) were obtained and further prepared into proper dimension for degradation and mechanical characterizations.
4. Conclusions
In many existing 3D printers, multiple syringes or material inlets are often required for the fabrication of devices of multiple parts each with varying mechanical properties. However, the addition of new materials is often very costly, and the printing parameters often require modifications between different materials. By combining PGSA with PEGDA and/or PCLDA, it is now possible to print biodegradable polymers with similar composition, but very different mechanical and degradation properties, in a continuous motion through instantaneous blending of the three polymers at various ratios. This work aims to provide a data that describes the tunable mechanical and degradation properties for the selection of biodegradable, photocurable polymer that may become useful in 3D printing. The development of instantaneous blending of prepolymers while printing is already under way, and is hoped to bring evolutionary change to many existing 3D printing systems.