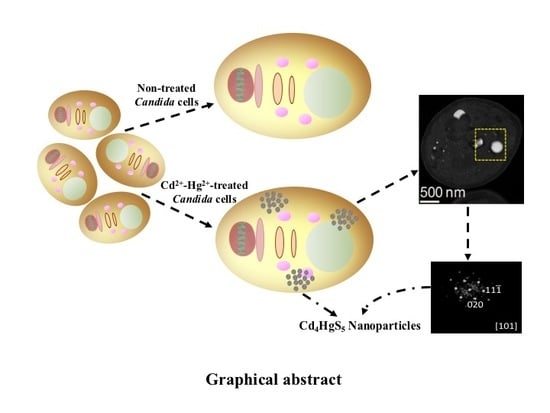
Synthesis of Bimetallic Nanoparticles of Cd4HgS5 by Candida Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Susceptibility Test
2.2. Growth Curves and Metal Uptake
2.3. Crystal Characterization
3. Results and Discussion
3.1. Susceptibility and Metal Uptake
3.2. Identification and Characterization of Biosynthetic Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef] [PubMed]
- Mallin, M.P.; Murphy, C.J. Solution–phase synthesis of sub–10 nm Au–Ag alloy nanoparticles. Nano Lett. 2002, 2, 1235–1237. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2017. [Google Scholar] [CrossRef]
- Toshima, N.; Yonezawa, T. Bimetallic nanoparticles—Novel materials for chemical and physical applications. New J. Chem. 1998, 22, 1179–1201. [Google Scholar] [CrossRef]
- Wang, A.; Liu, X.Y.; Mou, C.Y.; Zhang, T. Understanding the synergistic effects of gold bimetallic catalysts. J. Catal. 2013, 308, 258–271. [Google Scholar] [CrossRef] [Green Version]
- Sau, T.K.; Rogach, A.L. Nonspherical noblemetal nanoparticles: Colloid-chemical synthesis and morphology control. Adv. Mater. 2010, 22, 1781–1804. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Cruz, M.; Lucio-Hernández, D.; Martínez-Ángeles, I.; Demitri, N.; Polentarutti, M.; Rosales-Hoz, M.J.; Moreno, A. Biosynthesis of Micro- and Nano- Crystals of Pb (II), Hg (II) and Cd (II) Sulfides in Four Candida Species. A Comparative Study of in Vivo and in Vitro Approaches. Microb. Biotechnol. 2017, 10, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Lendave, S.A.; Karande, V.S.; Deshmukh, L.P. Optical and microstructural properties of chemically deposited mercury cadmium sulphide thin films. Surf. Eng. Appl. Electrochem. 2011, 46, 462–468. [Google Scholar] [CrossRef]
- Yang, J.; Hu, Y.; Luo, J.; Zhu, Y.H.; Yu, J.S. Highly Fluorescent, Near-Infrared-Emitting Cd2+-Tuned HgS Nanocrystals with Optical Applications. Langmuir 2015, 31, 3500–3509. [Google Scholar] [CrossRef] [PubMed]
- Hagler, A.; Mendonça-Hagler, L. Yeasts from marine and estuarine waters with different levels of pollution in the state of rio de janeiro, Brazil. Appl. Environ. Microbiol. 1981, 41, 173–178. [Google Scholar] [PubMed]
- López-Archilla, A.I.; González, A.E.; Terrón, M.C.; Amils, R. Ecological study of the fungal populations of the acidic Tinto river in southwestern Spain. Can. J. Microbiol. 2004, 50, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Suihko, M.L.; Hoekstra, E.S. Fungi present in some recycled fibre pulps and paperboards. Nordic Pulp Pap. Res. J. 1999, 14, 199–203. [Google Scholar] [CrossRef]
- Ramírez-Quijas, M.D.; Zazueta-Sandoval, R.; Obregón-Herrera, A.; López-Romero, E.; Cuéllar-Cruz, M. Effect of oxidative stress on cell wall morphology in four pathogenic Candida species. Mycol. Prog. 2015, 14. [Google Scholar] [CrossRef]
- Cuellar-Cruz, M.; Briones-Martin-Del-Campo, M.; Canas-Villamar, I.; Montalvo-Arredondo, J.; Riego-Ruiz, L.; Castano, I.; Penas, A. High resistance to oxidative stress in the fungal pathogen Candida glabrata is mediated by a single catalase, Cta1p, and is controlled by the transcription factors Yap1p, kn7p, Msn2p, and Msn4p. Eukaryot. Cell 2008, 7, 814–825. [Google Scholar] [PubMed]
- Serrano-Fujarte, I.; Lopez-Romero, E.; Reyna-Lopez, G.E.; Martinez-Gamez, M.A.; Vega-Gonzalez, A.; Cuellar-Cruz, M. Influence of culture media on biofilm formation by Candida species and response of sessile cells to antifungals and oxidative stress. Biomed. Res. Int. 2015, 2015, 783639. [Google Scholar] [PubMed]
- Nordberg, M.; Nordberg, G. Toxicological aspects of metallothionein. Cell. Mol. Biol. 2000, 46, 451–463. [Google Scholar] [PubMed]
- Nies, D.H.; Silver, S. Ion efflux systems involved in bacterial metal resistances. J. Ind. Microbiol. 1995, 14, 186–199. [Google Scholar] [PubMed]
- Goyer, R.A. Response to Comments by Professor Duffus regarding Chapter 23, Toxic Effects of Metals. In Cassarett and Doull’s Toxicology; McGraw Hill: New York, NY, USA, 2001; pp. 265–266. [Google Scholar]
- Méndez-Armenta, M.; Ríos, C. Cadmium neurotoxicity. Environ. Toxicol. Pharmacol. 2007, 23, 350–358. [Google Scholar] [PubMed]
- Pan-Hou, H.S.; Imura, N. Involvement of mercury methylation in microbial mercury detoxication. Arch. Microbiol. 1982, 131, 176–177. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Núñez, A.; González, G.; Romero-Ibarra, J.E.; Vega-González, A.; Cruz-Jiménez, G.; Hernández-Cristóbal, O.; Zárraga-Núñez, R.A.; Obregón-Herrera, A.; López-Romero, E.; Pedraza-Reyes, M.; et al. Synthesis of Bimetallic Nanoparticles of Cd4HgS5 by Candida Species. Crystals 2019, 9, 61. https://doi.org/10.3390/cryst9020061
Romero-Núñez A, González G, Romero-Ibarra JE, Vega-González A, Cruz-Jiménez G, Hernández-Cristóbal O, Zárraga-Núñez RA, Obregón-Herrera A, López-Romero E, Pedraza-Reyes M, et al. Synthesis of Bimetallic Nanoparticles of Cd4HgS5 by Candida Species. Crystals. 2019; 9(2):61. https://doi.org/10.3390/cryst9020061
Chicago/Turabian StyleRomero-Núñez, Araceli, Gonzalo González, Josué E. Romero-Ibarra, Arturo Vega-González, Gustavo Cruz-Jiménez, Orlando Hernández-Cristóbal, Ramón A. Zárraga-Núñez, Armando Obregón-Herrera, Everardo López-Romero, Mario Pedraza-Reyes, and et al. 2019. "Synthesis of Bimetallic Nanoparticles of Cd4HgS5 by Candida Species" Crystals 9, no. 2: 61. https://doi.org/10.3390/cryst9020061