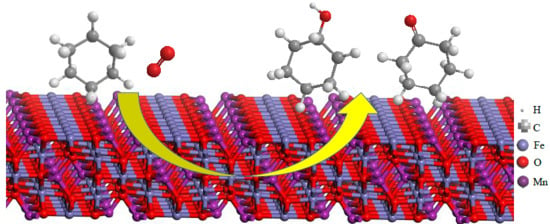
Synergetic Effect of Calcium Doping on Catalytic Activity of Manganese Ferrite: DFT Study and Oxidation of Hydrocarbon
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of the Catalysts
2.2. Characterization of Catalysts
2.3. DFT Study of the Catalyst
2.4. Oxidation Reaction
3. Results and Discussion
3.1. Physical Characterization of the Catalysts
3.2. Screening of the Catalyst
3.3. Kinetics Studies
3.3.1. Effect of Oxygen Partial Pressure
3.3.2. Effect of Initial Substrate Volume
3.3.3. Separation and Recyclability of MnFe2O4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhenyu, L.; Guangliang, X.; Yalin, Z. Microwave assisted low temperature synthesis of MnZn ferrite nanoparticles. Nanoscale Res. Lett. 2007, 2, 40. [Google Scholar] [CrossRef] [Green Version]
- Koleva, K.V.; Velinov, N.I.; Tsoncheva, T.S.; Mitov, I.G.; Kunev, B.N. Preparation, structure and catalytic properties of ZnFe2O4. Bulg. Chem. Commun. 2013, 45, 434–439. [Google Scholar]
- Chavan, A.R.; Birajdar, S.D.; Chilwar, R.R.; Jadhav, K.M. Structural, morphological, optical, magnetic and electrical properties of Al3+ substituted nickel ferrite thin films. J. Alloys Compd. 2018, 735, 2287–2297. [Google Scholar] [CrossRef]
- Devi, E.C.; Soibam, I. Structural and optical characterization of MnFe2O4 nanoparticles. Adv. Mater. Proc. 2017, 2, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Hassandoost, R.; Pouran, S.R.; Khataee, A.; Orooji, Y.; Joo, S.W. Hierarchically structured ternary heterojunctions based on Ce3+/Ce4+ modified Fe3O4 nanoparticles anchored onto graphene oxide sheets as magnetic visible-light-active photocatalysts for decontamination of oxytetracycline. J. Hazard. Mater. 2019, 376, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Ullah, Z.; Atiq, S.; Naseem, S. Influence of Pb doping on structural, electrical and magnetic properties of Sr-hexaferrites. J. Alloys Compd. 2013, 555, 263–267. [Google Scholar] [CrossRef]
- Tatarchuk, T.R.; Paliychuk, N.D.; Bououdina, M.; Al-Najar, B.; Pacia, M.; Macyk, W.; Shyichuk, A. Effect of cobalt substitution on structural, elastic, magnetic and optical properties of zinc ferrite nanoparticles. J. Alloys Compd. 2018, 15, 1256–1266. [Google Scholar] [CrossRef]
- Monsef, K.Z.; Najafi, N. Magnetic solid-phase extraction to preconcentrate trace amounts of gold (III) using nickel ferrite magnetic nanoparticles. Int. J. Environ. Anal. Chem. 2017, 97, 1237–1252. [Google Scholar] [CrossRef]
- Joshi, S.; Kumar, M.; Chhoker, S.; Srivastava, G.; Jewariya, M.; Singh, V.N. Structural, magnetic, dielectric and optical properties of nickel ferrite nanoparticles synthesized by co-precipitation method. J. Mol. Struct. 2014, 1076, 55–62. [Google Scholar] [CrossRef]
- Lu, H.C.; Chang, J.E.; Vong, W.W.; Chen, H.T.; Chen, Y.L. Porous ferrite synthesis and catalytic effect on benzene degradation. Int. J. Phys. Sci. 2011, 6, 855–865. [Google Scholar]
- Ustundağ, M.; Aslan, M. Electronic and Magnetic Properties of Ca-Doped Mn-Ferrite. Acta Phys. Pol. A 2016, 130, 362–364. [Google Scholar] [CrossRef]
- Perumal, S.L.; Hemalatha, P.; Alagara, M.; Pandiyaraj, K.N. Investigation of structural, optical and photocatalytic properties of Sr doped Zno nanoparticles. Int. J. Phys. Sci. 2015, 4, 1–13. [Google Scholar]
- Kuo, S.L.; Wu, N.L. Electrochemical characterization on MnFe2O4/carbon black composite aqueous supercapacitors. J. Power Sources 2006, 162, 1437–1443. [Google Scholar] [CrossRef]
- Menini, L.; Pereira, M.C.; Parreira, L.A.; Fabris, J.D.; Gusevskaya, E.V. Cobalt-and manganese-substituted ferrites as efficient single-site heterogeneous catalysts for aerobic oxidation of monoterpenic alkenes under solvent-free conditions. J. Catal. 2008, 254, 355–364. [Google Scholar] [CrossRef]
- Valdés, S.T.; Valle, P.; Alvarez, S.; Marbán, G.; Fuertes, A.B. Manganese ferrite nanoparticles synthesized through a nanocasting route as a highly active Fenton catalyst. Catal. Commun. 2007, 8, 2037–2042. [Google Scholar] [CrossRef]
- Zhang, Y.; Elfman, M.; Winzell, T.; Whitlow, H.J. Characterisation of ferromagnetic magnetic storage media surfaces by complementary particle induced X-ray analysis and time of flight-energy dispersive elastic recoil detection analysis. Nucl. Instrum. Methods Physics Res. Sect. B Beam Interact. Mater. At. 1999, 150, 548–553. [Google Scholar] [CrossRef]
- Xiao, H.M.; Liu, X.M.; Fu, S.Y. Synthesis, magnetic and microwave absorbing properties of core-shell structured MnFe2O4/TiO2 nanocomposites. Compos. Sci. Technol. 2006, 66, 2003–2008. [Google Scholar] [CrossRef]
- Rocha, S.T.A. Sensors and biosensors based on magnetic nanoparticles. Trac Trend Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Shah, S.; Asdi, M.H.; Hashmi, M.U.; Umar, M.F.; Awan, S.U. Thermo-responsive copolymer coated MnFe2O4 magnetic nanoparticles for hyperthermia therapy and controlled drug delivery. Mater. Chem. Phys. 2012, 137, 365–371. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Zhou, L.; Ma, W.; Zhang, D.; Ren, F.; Yan, H.; Qiu, G.; Liu, X. A facile solvothermal synthesis and magnetic properties of MnFe2O4 spheres with tunable sizes. J. Am. Ceram. Soc. 2012, 95, 3569–3576. [Google Scholar] [CrossRef]
- Alves, C.R.; Aquino, R.; Depeyrot, J.; Tourinho, F.A.; Dubois, E.; Perzynski, R. Superparamagnetic relaxation evidences large surface contribution for the magnetic anisotropy of MnFe2O4 nanoparticles of ferrofluids. J. Mater. Sci. 2007, 42, 2297–2303. [Google Scholar] [CrossRef]
- Dolgykh, L.Y.; Stolyarchuk, I.L.; Vasylenko, I.V.; Pyatnitsky, Y.I.; Strizhak, P.E. Influence of the Composition of Nanosized MFe2O4 Spinels (M = Ni, Co, Mn) on Their Catalytic Properties in the Steam Reforming of Ethanol. Theor. Exp. Chem. 2013, 1, 185–192. [Google Scholar] [CrossRef]
- Sahoo, B.; Sahu, S.K.; Nayak, S.; Dhara, D.; Pramanik, P. Fabrication of magnetic mesoporous manganese ferrite nanocomposites as efficient catalyst for degradation of dye pollutants. Catal. Sci. Technol. 2012, 2, 1367–1374. [Google Scholar] [CrossRef]
- Dossumov, K.; Yergazieva, G.Y.; Myltykbaieva, L.K.; Asanov, N.A. Effect of Co, Ce, and La Oxides as Modifying Additives on the Activity of an NiO/γ-Al2O3 Catalyst in the Oxidation of Methane to Give Synthesis Gas. Theor. Exp. Chem. 2016, 52, 119–122. [Google Scholar] [CrossRef]
- Zakharchenko, N.I. Catalytic Properties of the Fe2O3–MnO System for Ammonia Oxidation. Kinet. Catal. 2001, 42, 679–685. [Google Scholar] [CrossRef]
- Lou, J.C.; Tu, Y.J. Incinerating volatile organic compounds with ferrospinel catalyst MnFe2O4: An example with isopropyl alcohol. J. Air Waste Manag. 2005, 55, 1809–1815. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wen, W.; Kong, L.; Tian, S.; Ding, F.; Xiong, Y. Magnetically separable and durable MnFe2O4 for efficient catalytic ozonation of organic pollutants. Ind. Eng. Chem. Res. 2014, 53, 6297–6306. [Google Scholar] [CrossRef]
- Xu, B.; Bhawe, Y.; Davis, M.E. Spinel metal oxide-alkali carbonate-based, low-temperature thermochemical cycles for water splitting and CO2 reduction. Chem. Mater. 2013, 25, 1564–1571. [Google Scholar] [CrossRef] [Green Version]
- Martins, N.; Martins, L.; Amorim, C.; Amaral, V.; Pombeiro, A. Solvent-free microwave-induced oxidation of alcohols catalyzed by ferrite magnetic nanoparticles. Catalysts 2017, 7, 222. [Google Scholar] [CrossRef] [Green Version]
- Bhat, P.B.; Bhat, B.R. Magnetically retrievable nickel hydroxide functionalised A Fe2O 4 (A = Mn, Ni) spinel nanocatalyst for alcohol oxidation. Appl. Nanosci. 2016, 6, 425–435. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.J.; Wischert, R.; Xue, K.; Zheng, Y.T.; Albela, B.; Bonneviot, L.; Clacens, J.M.; De, C.F.; Pera, T.M.; Wu, P. Highly selective liquid-phase oxidation of cyclohexane to KA oil over Ti-MWW catalyst: Evidence of formation of oxyl radicals. ACS Catal. 2013, 4, 53–62. [Google Scholar] [CrossRef]
- Ramanathan, A.; Hamdy, M.S.; Parton, R.; Maschmeyer, T.; Jansen, J.C.; Hanefeld, U. Co-TUD-1 catalysed aerobic oxidation of cyclohexane. Appl. Catal. A Gen. 2009, 355, 78–82. [Google Scholar] [CrossRef]
- Lü, G.; Zhao, R.; Qian, G.; Qi, Y.; Wang, X.; Suo, J. A highly efficient catalyst Au/MCM-41 for selective oxidation cyclohexane using oxygen. Catal. Lett. 2004, 97, 115–118. [Google Scholar] [CrossRef]
- Schuchardt, U.; Cardoso, D.; Sercheli, R.; Pereira, R.; Da Cruz, R.S. Cyclohexane oxidation continues to be a challenge. Appl. Catal. A Gen. 2001, 211, 1–17. [Google Scholar] [CrossRef]
- George, K.; Sugunan, S. Catalytic oxidation of cyclohexane over Cu-Zn-Cr ternary spinel systems. React. Kinet. Catal. Lett. 2008, 94, 253. [Google Scholar] [CrossRef] [Green Version]
- Rana, R.K.; Viswanathan, B. Mo incorporation in MCM-41 type zeolite. Catal. Lett. 1998, 52, 25–29. [Google Scholar] [CrossRef]
- Liu, Y.; Tsunoyama, H.; Akita, T.; Xie, S.; Tsukuda, T. Aerobic oxidation of cyclohexane catalyzed by size-controlled Au clusters on hydroxyapatite: Size effect in the sub-2 nm regime. ACS Catal. 2010, 1, 2–6. [Google Scholar] [CrossRef]
- Jhansi, M.; Kishore, L.; Kumar, A. Heteronuclear macrocyclinc iron–copper complex catalyst covalently bonded to modified alumina catalyst for oxidation of cyclohexane. Int. Eng. Chem. Res. 2007, 46, 4787–4798. [Google Scholar]
- Zhu, K.; Hu, J.; Richards, R. Aerobic oxidation of cyclohexane by gold nanoparticles immobilized upon mesoporous silica. Catal. lett. 2005, 100, 195–199. [Google Scholar] [CrossRef]
- Ebadi, A.; Safari, N.; Peyrovi, M.H. Aerobic oxidation of cyclohexane with γ-alumina supported metallophthalocyanines in the gas phase. Appl. Catal. A Gen. 2007, 321, 135–139. [Google Scholar] [CrossRef]
- Da Cruz, R.S.; Silva, J.M.; Arnold, U.; Schuchardt, U. Catalytic activity and stability of a chromium containing silicate in liquid phase cyclohexane oxidation. J. Mol. Cat. A Chem. 2001, 171, 251–257. [Google Scholar] [CrossRef]
- Aijun, H.; Juanjuan, L.; Mingquan, Y.; Yan, L.; Xinhua, P. Preparation of nano-MnFe2O4 and its catalytic performance of thermal decomposition of ammonium perchlorate. Chin. J. Chem. Eng. 2011, 19, 1047–1051. [Google Scholar]
- Blaha, P.; Schwarz, K.; Madsen, G.K.; Kvasnicka, D.; Luitz, J. WIEN2K, an Augmented Plane Wave+ Local Orbitals Program for Calculating Crystal Properties; Vienna University of Technology: Vienna, Austria, 2001. [Google Scholar]
- Tran, F.; Blaha, P. Accurate band gaps of semiconductors and insulators with a semilocal exchange-correlation potential. Phys. Rev. Lett. 2009, 102, 226401. [Google Scholar] [CrossRef] [Green Version]
- Bellusci, M.; La, B.A.; Seralessandri, L.; Padella, F.; Piozzi, A.; Varsano, F. Preparation of albumin–ferrite superparamagnetic nanoparticles using reverse micelles. Polym. Int. 2009, 58, 142–147. [Google Scholar] [CrossRef]
- Rodionova, L.I.; Smirnov, A.V.; Borisova, N.E.; Khrustalev, V.N.; Moiseeva, A.A.; Grünert, W. Binuclear cobalt complex with Schiff base ligand: Synthesis, characterization and catalytic properties in partial oxidation of cyclohexane. Inorg. Chim. Acta. 2012, 392, 221–228. [Google Scholar] [CrossRef]
- Vignesh, R.H.; Sankar, K.V.; Amaresh, S.; Lee, Y.S.; Selvan, R.K. Synthesis and characterization of MnFe2O4 nanoparticles for impedometric ammonia gas sensor. Sens. Actuators B Chem. 2015, 220, 50–58. [Google Scholar] [CrossRef]
- Kooti, M.; Sedeh, A.N. Glycine-assisted fabrication of zinc and manganese ferrite nanoparticles. Sci. Iran. 2012, 19, 930–933. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Yin, Z.; Wang, H.; Li, J.; Liang, X.; Kang, J.; He, B. Controllable oxidation of cyclohexane to cyclohexanol and cyclohexanone by a nano-MnOx/Ti electrocatalytic membrane reactor. J. Catal. 2015, 329, 187–194. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Liu, N.; Liu, Y.; Huang, H.; Kang, Z. Total photocatalysis conversion from cyclohexane to cyclohexanone by C3N4/Au nanocomposites. Green Chem. 2014, 16, 4559–4565. [Google Scholar] [CrossRef]
- Hou, Z.; Yokota, O.; Tanaka, T.; Yashima, T. Characterization of Ca-promoted Ni/α-Al2O3 catalyst for CH4 reforming with CO2. Appl. Catal. A Gen. 2003, 253, 381–387. [Google Scholar] [CrossRef]
- Rizvi, S.B.; Yildirimer, L.; Ghaderi, S.; Ramesh, B.; Seifalian, A.M.; Keshtgar, M. A novel POSS-coated quantum dot for biological application. Int. J. Nanomed. 2012, 7, 3915–3927. [Google Scholar]
- Ilyas, M.; Sadiq, M. Liquid-Phase Aerobic Oxidation of Benzyl Alcohol Catalyzed by Pt/ZrO2. Chem. Eng. Technol. 2007, 30, 1391–1397. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Lapkin, A.A.; Plucinski, P.K.; Friedrich, J.M.; Walsh, F.C. TiO2 nanotube-supported ruthenium (III) hydrated oxide: A highly active catalyst for selective oxidation of alcohols by oxygen. J. Catal. 2005, 235, 10–17. [Google Scholar] [CrossRef]











| S. No | Catalyst | Reactant | Reaction Conditions | * Productivity (mmolg−1h−1) | Ref |
|---|---|---|---|---|---|
| 1 | Ti-MWW | bCH | 80 °C, 1 h, 4 g of CH, 0.10 g of catalyst, TBHP | 50.85 b | [31] |
| 2 | C3N4/Au | bCH | 60 °C, 24 h, 10 mL of CH, 0.5 g of catalyst, 200 mL ultra-pure water, Xe-lamp | 0.812 b | [50] |
| 3 | MnFe2O4 | abcCH | 80 °C, 3.5 h, 20 mL of CH, 0.15 g of catalyst, O2 | 366.17 a 241.17 b 219.17 c | This study |
| 4 | Ca-MnFe2O4 | abcCH | 80 °C, 3.5 h, 20 mL of CH, 0.15 g of catalyst, O2 | 379.38 a 285.31 b 247.31 c | This study |
| S. No | Catalyst | Productivity * | Synergistic Effect of Mn |
|---|---|---|---|
| 1 | Iron oxide | 101.52 | 264.65 |
| 2 | MnFe2O4 | 366.17 | |
| 3 | 20% > Ca-MnFe2O4 | 379.38 | Enhancing Effect of Ca on MnFe2O4 |
| 13.21 | |||
| 4 | 50% ≤ Ca-MnFe2O4 | 359.31 | Retarding Effect of Ca on MnFe2O4 |
| 6.79 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, Z.; Sadiq, S.; Sadiq, M.; Ali, M.; Saeed, K.; Ur Rehman, N.; Ilyas, M.; Ullah, S.; Ullah Jan, S.; Ahmad, I.; et al. Synergetic Effect of Calcium Doping on Catalytic Activity of Manganese Ferrite: DFT Study and Oxidation of Hydrocarbon. Crystals 2020, 10, 335. https://doi.org/10.3390/cryst10040335
Iqbal Z, Sadiq S, Sadiq M, Ali M, Saeed K, Ur Rehman N, Ilyas M, Ullah S, Ullah Jan S, Ahmad I, et al. Synergetic Effect of Calcium Doping on Catalytic Activity of Manganese Ferrite: DFT Study and Oxidation of Hydrocarbon. Crystals. 2020; 10(4):335. https://doi.org/10.3390/cryst10040335
Chicago/Turabian StyleIqbal, Zahoor, Saima Sadiq, Muhammad Sadiq, Muhammad Ali, Khalid Saeed, Najeeb Ur Rehman, Mohammad Ilyas, Saif Ullah, Saeed Ullah Jan, Iftikhar Ahmad, and et al. 2020. "Synergetic Effect of Calcium Doping on Catalytic Activity of Manganese Ferrite: DFT Study and Oxidation of Hydrocarbon" Crystals 10, no. 4: 335. https://doi.org/10.3390/cryst10040335