Effects of Various Inhibitors on the Nucleation of Calcium Oxalate in Synthetic Urine
Abstract
:1. Introduction
2. Experimental
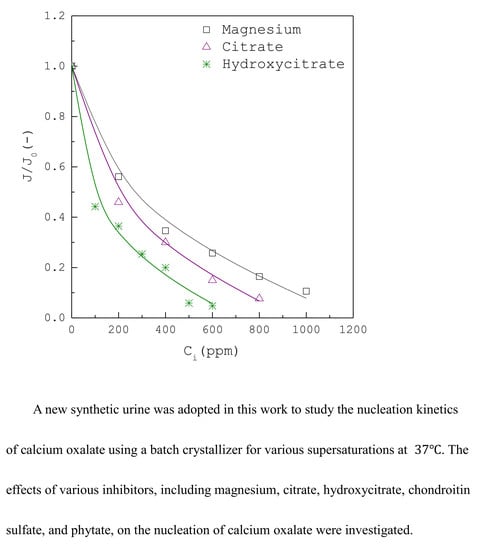
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Notation
| Nucleation kinetic parameter | |
| Concentration of inhibitor | |
| Nucleation rate | |
| Minimum detectable number density of nuclei | |
| Boltzmann constant | |
| Molar mass | |
| Avogadro number | |
| Supersaturation | |
| Temperature | |
| Induction time | |
| Interfacial energy | |
| Crystal density | |
| Volume of the solu te molecule |
References
- Scales, C.D.; Smith, A.C.; Hanley, J.M.; Saigal, C.S. Prevalence of kidney stones in the United States. Eur. Urol. 2012, 62, 160–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, Y.; Abram, V.; Coe, F.L. Isolation of calcium oxalate crystal growth inhibitor from rat kidney and urine. Am. J. Physiol. 1984, 247, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Ogbuji, L.U.; Batich, C.D. Ultrastructure of whewellite kidney stones: Electron-analytical investigation. J. Ultrastruct Res. 1985, 90, 1–8. [Google Scholar] [CrossRef]
- Kaloustian, J.; El-Moselhy, T.F.; Portugal, T.F. Determination of calcium oxalate (mono-and dihydrate) in mixtures with magnesium ammonium phosphate or uric acid: The use of simultaneous thermal analysis in urinary calculi. Clin. Chim. Acta 2003, 334, 117–129. [Google Scholar] [CrossRef]
- Opalko, F.J.; Adair, J.H.; Khan, S.R. Heterogeneous nucleation of calcium oxalate trihydrate in artificial urine by constant composition. J. Cryst. Growth 1997, 181, 410–417. [Google Scholar] [CrossRef]
- Rabinovich, Y.I.; Esayanur, M.; Daosukho, S.; Byer, K.J.; El-Shall, H.E.; Khan, S.R. Adhesion force between calcium oxalate monohydrate crystal and kidney epithelial cells and possible relevance for kidney stone formation. J. Colloid Interface Sci. 2006, 300, 131–140. [Google Scholar] [CrossRef]
- Li, M.K.; Blacklock, N.J.; Garside, J. Effects of magnesium on calcium oxalate crystallization. J. Urol. 1985, 133, 123–125. [Google Scholar] [CrossRef]
- Pak, C.Y. Citrate and renal calculi: New insights and future directions. Am. J. Kidney Dis. 1991, 17, 420–425. [Google Scholar] [CrossRef]
- Grases, F.; Isern, B.; Sanchis, P.; Perello, J.; Torres, J.J.; Costa-Bauza, A. Phytate acts as an inhibitor in formation of renal calculi. Front Biosci. 2007, 12, 2580–2587. [Google Scholar] [CrossRef]
- Chung, J.; Granja, I.; Taylor, M.G.; Mpourmpakis, G.; Asplin, J.R.; Rimer, J.D. Molecular modifiers reveal a mechanism of pathological crystal growth inhibition. Nature. 2016, 536, 446–450. [Google Scholar] [CrossRef]
- Rodgers, A.L.; Jackson, G.E. Determination of thermodynamic parameters for complexation of calcium and magnesium with chondroitin sulfate isomers using isothermal titration calorimetry: Implications for calcium kidney-stone research. J. Cryst. Growth 2017, 463, 14–18. [Google Scholar] [CrossRef]
- Kim, D.; Rimer, J.D.; Asplin, J.R. Hydroxycitrate: A potential new therapy for calcium urolithiasis. Urolithiasis 2019, 47, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Coe, F.L. Acidic peptide and polyribonucleotide crystal growth inhibitors in human urine. Am. J. Physiol. 1977, 233, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Drach, G.W.; Randolph, A.D.; Miller, J.D. Inhibition of calcium oxalate dihydrate crystallization by chemical modifiers. I. Pyrophosphate and methylene blue. J. Urol. 1978, 119, 99–103. [Google Scholar] [CrossRef]
- Hallson, P.C.; Rose, G.A. Uromucoids and urinary stone formation. Lancet 1979, 1, 1000–1002. [Google Scholar] [CrossRef]
- Randolph, A.D.; Drach, G.W. Some measurements of calcium oxalate nucleation and growth rates in urine-like liquors. J. Cryst. Growth 1981, 53, 195–201. [Google Scholar] [CrossRef]
- Robertson, W.G.; Scurr, D.S. Factors influencing the crystallization of calcium oxalate-a critique. J. Cryst. Growth 1981, 53, 182–194. [Google Scholar] [CrossRef]
- Ryall, R.L.; Harnett, R.M.; Marshall, V.R. The effect of urine, pyrophosphate, citrate, magnesium and glycosaminoglycans on the growth and aggregation of calcium oxalate crystals in vitro. Clin. Chim. Acta 1981, 112, 349–356. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Abram, V.; Kezdy, F.J.; Kaiser, E.T.; Coe, F.L. Purification and characterization of the principal inhibitor of calcium oxalate crystal growth in human urine. J. Biol. Chem. 1983, 258, 12594–12600. [Google Scholar]
- Robertson, W.G.; Scurr, D.S.; Sergeant, V.J. Ionic and macromolecular modifiers of crystallization of calcium salts in urine. Fortschr. Urol. Nephrol. 1985, 23, 1–11. [Google Scholar]
- Robertson, W.G.; Scurr, D.S. Modifiers of calcium oxalate crystallization found in urine. I. Studies with a continuous crystallizer using an artificial urine. J. Urol. 1986, 86, 1322–1326. [Google Scholar] [CrossRef]
- Grases, F.; Rodriguez, A.; Costa-Bauza, A. Efficacy of mixtures of magnesium, citrate and phytate as calcium oxalate crystallization inhibitors in urine. J. Urol. 2015, 194, 812–819. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization; Butterworth-Heinemann: Oxford, UK, 1993. [Google Scholar]
- Chutipongtanate, S.; Thongboonkerd, V. Systematic comparisons of artificial urine formulas for in vitro cellular study. Anal. Biochem. 2010, 402, 110–112. [Google Scholar] [CrossRef]
- Finlayson, B. Calcium stones: Some physical and clinical aspects, Chapter 10. In Calcium Metabolism in Renal Failure and Nephrolithiasis; David, D.S., Ed.; John Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Kubota, N. A new interpretation of metastable zone widths measured for unseeded solutions. J. Cryst. Growth 2008, 310, 629–634. [Google Scholar] [CrossRef]
- Kobari, M.; Kubota, N.; Hirasawa, I. Deducing primary nucleation parameters from metastable zone width and induction time data determined with simulation. CrystEngComm 2013, 15, 1199–1209. [Google Scholar] [CrossRef]
- Shiau, L.D.; Lu, T.S. A model for determination of the interfacial energy from the induction time or metastable zone width data based on turbidity measurements. CrystEngComm 2014, 16, 9743–9752. [Google Scholar] [CrossRef]
- Shiau, L.D. Comparison of the interfacial energy and pre-exponential factor calculated from the induction time and metastable zone width data based on classical nucleation theory. J. Cryst. Growth 2016, 450, 50–55. [Google Scholar] [CrossRef]
- Shiau, L.D. Determination of the nucleation and growth kinetics of aqueous L-glycine solutions from the turbidity induction time data. Crystals 2018, 8, 403. [Google Scholar] [CrossRef] [Green Version]
- Mersmann, A.; Bartosch, K. How to predict the metastable zone width. J. Cryst. Growth 1998, 183, 240–250. [Google Scholar] [CrossRef]
- Lindenberg, C.; Mazzotti, M. Effect of temperature on the nucleation kinetics of α L-glutamic acid. J. Cryst. Growth 2009, 311, 1178–1184. [Google Scholar] [CrossRef]
- Antinozzi, P.A.; Brown, C.M.; Purich, D.L. Calcium oxalate monohydrate crystallization: Citrate inhibition of nucleation and growth steps. J. Cryst. Growth 1992, 125, 215–222. [Google Scholar] [CrossRef]
- Brown, C.M.; Ackermann, D.K.; Purich, D.L.; Finlayson, B. Nucleation of calcium oxalate monohydrate: Use of turbidity measurements and computer-assisted simulations in characterizing early events in crystal formation. J. Cryst. Growth 1991, 108, 455–464. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frolich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. Suppl. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sheikholeslami, R.; Doherty, W.O.S. The effects of silica and sugar on the crystallographic and morphological properties of calcium oxalate. J. Cryst. Growth 2004, 265, 592–603. [Google Scholar] [CrossRef]









| Composition | Concentration (mM) |
|---|---|
| Solution 1 (100 mL): | |
| Na2SO4·10H2O | 9.67 |
| NH4Cl | 43.37 |
| KCl | 81.30 |
| CaCl2 | 5.00 |
| Solution 2 (100 mL): | |
| NaH2PO4·2H2O | 7.73 |
| Na2HPO4·12H2O | 7.82 |
| NaCl | 111.54 |
| Urea | 200 |
| Uric acid | 1.00 |
| Creatinine | 4.00 |
| Solution 3 (3 mL): | |
| Na2C2O4 | 25.6, 32.4, 40.0, 57.6 |
| Composition | Concentration (mM) |
|---|---|
| Na2SO4·10H2O | 4.76 |
| NH4Cl | 21.36 |
| KCl | 40.05 |
| CaCl2 | 2.46 |
| NaH2PO4·2H2O | 3.81 |
| Na2HPO4·12H2O | 3.85 |
| NaCl | 54.95 |
| Urea | 98.52 |
| Uric acid | 0.49 |
| Creatinine | 1.97 |
| Na2C2O4 | 0.38, 0.48, 0.59, 0.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-C.; Lin, Y.-H.; Shiau, L.-D. Effects of Various Inhibitors on the Nucleation of Calcium Oxalate in Synthetic Urine. Crystals 2020, 10, 333. https://doi.org/10.3390/cryst10040333
Hsu Y-C, Lin Y-H, Shiau L-D. Effects of Various Inhibitors on the Nucleation of Calcium Oxalate in Synthetic Urine. Crystals. 2020; 10(4):333. https://doi.org/10.3390/cryst10040333
Chicago/Turabian StyleHsu, Yu-Chao, Yi-Hsuan Lin, and Lie-Ding Shiau. 2020. "Effects of Various Inhibitors on the Nucleation of Calcium Oxalate in Synthetic Urine" Crystals 10, no. 4: 333. https://doi.org/10.3390/cryst10040333