Surfactant-Free Synthesis of Reduced Graphene Oxide Supported Well-Defined Polyhedral Pd-Pt Nanocrystals for Oxygen Reduction Reaction
Abstract
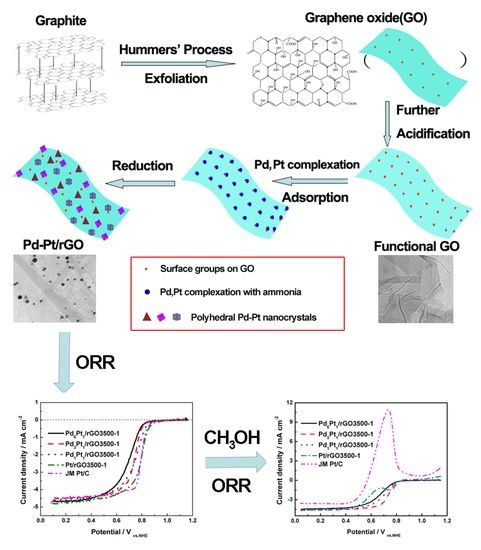
:1. Introduction
2. Experimental
2.1. Synthesis of Reduced Graphene Oxides Anchored Pd-Pt Nanocrystals
2.2. Physicochemical Characterizations of the Pd-Pt/rGO Samples
2.3. Fabrication of Working Electrode and Electrochemical Measurement
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Gao, W.; Liu, Z.; Zeng, Z.; Liu, Y.; Giroux, M.; Chi, M.; Wang, G.; Greeley, J.; Pan, X.; et al. Core–shell Nanostructured Cobalt-Platinum Electrocatalysts with Enhanced Durability. ACS Catal. 2018, 8, 35–42. [Google Scholar] [CrossRef]
- Li, J.; Xi, Z.; Pan, Y.T.; Spendelow, J.S.; Duchesne, P.N.; Su, D.; Li, Q.; Yu, C.; Yin, Z.; Shen, B.; et al. Fe Stabilization by Intermetallic L10-FePt and Pt Catalysis Enhancement in L10-FePt/Pt Nanoparticles for Efficient Oxygen Reduction Reaction in Fuel Cells. J. Am. Chem. Soc. 2018, 140, 2926–2932. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Iqbal, M.; Lin, J.; Luo, X.; Jiang, B.; Malgras, V.; Wu, K.C.W.; Kim, J.; Yamauchi, Y. Electrochemical Deposition: An Advanced Approach for Templated Synthesis of Nanoporous Metal Architectures. Acc. Chem. Res. 2018, 511, 764–1773. [Google Scholar] [CrossRef] [PubMed]
- Asset, T.; Job, N.; Busby, Y.; Crisci, A.; Martin, V.; Stergiopoulos, V.; Bonnaud, C.; Serov, A.; Atanassov, P.; Chattot, R.; et al. Porous Hollow PtNi/C Electrocatalysts: Carbon Support Considerations to Meet Performance and Stability Requirements. ACS Catal. 2018, 8, 893–903. [Google Scholar] [CrossRef]
- Li, C.L.; Tan, H.B.; Lin, J.J.; Luo, X.L.; Wang, S.P.; You, J.; Kang, Y.H.; Bando, Y.; Yamauchi, Y.; Kim, J. Emerging Pt-based electrocatalysts with highly open nanoarchitectures for boosting oxygen reduction reaction. Nano Today 2018, 21, 91–105. [Google Scholar] [CrossRef]
- Wang, X.; Choi, S.; Roling, L.T.; Luo, M.; Ma, C.; Zhang, L.; Chi, M.; Liu, J.; Xie, Z.; Herron, J.A.; et al. Palladium-platinum core–shell icosahedra with substantially enhanced activity and durability towards oxygen reduction. Nat. Commun. 2015, 6, 7594. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Choi, S.; Lu, N.; Roling, L.T.; Herron, J.A.; Zhang, L.; Park, J.; Wang, J.; Kim, M.J.; Xie, Z.; et al. Atomic Layer-by-Layer Deposition of Pt on Pd Nanocubes for Catalysts with Enhanced Activity and Durability toward Oxygen Reduction. Nano Lett. 2014, 14, 3570–3576. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt Bimetallic Nanodendrites with High Activity for Oxygen Reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.W.; Ko, A.R.; Kim, D.Y.; Han, S.B.; Park, K.W. Octahedral Pt-Pd alloy catalysts with enhanced oxygen reduction activity and stability in proton exchange membrane fuel cells. RSC Adv. 2012, 2, 1119–1125. [Google Scholar] [CrossRef]
- He, W.; Chen, M.; Zou, Z.; Li, Z.; Zhang, X.; Jin, S.A.; You, D.J.; Pak, C.; Yang, H. Oxygen reduction on Pd3Pt1 bimetallic nanoparticles highly loaded on different carbon supports. Appl. Catal. B Environ. 2010, 97, 347–353. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, F.; Yu, S.; Li, Z.; Zhao, Y. Surfactant-free synthesis of highly methanol-tolerant, polyhedral Pd-Pt nanocrystallines for oxygen reduction reaction. J. Power Sources 2013, 239, 374–381. [Google Scholar] [CrossRef]
- Mazumder, V.; Chi, M.; More, K.L.; Sun, S. Core/Shell Pd/FePt Nanoparticles as an Active and Durable Catalyst for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2010, 132, 7848–7849. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, C.; Janiak, C. Naked metal nanoparticles from metal carbonyls in ionic liquids: Easy synthesis and stabilization. Coord. Chem. Rev. 2011, 255, 2039–2057. [Google Scholar] [CrossRef]
- Liu, Y.; Chi, M.; Mazumder, V.; More, K.L.; Soled, S.; Henao, J.D.; Sun, S. Composition-Controlled Synthesis of Bimetallic PdPt Nanoparticles and Their Electro-oxidation of Methanol. Chem. Mater. 2011, 23, 4199–4203. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, V.; Lee, Y.; Sun, S. Recent Development of Active Nanoparticle Catalysts for Fuel Cell Reactions. Adv. Funct. Mater. 2010, 20, 1224–1231. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.; Yu, T.; Camargo, P.H.C.; Xia, Y. Nucleation and Growth Mechanisms for Pd-Pt Bimetallic Nanodendrites and Their Electrocatalytic Properties. Nano Res. 2010, 3, 69–80. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.; Tao, J.; Camargo, P.H.C.; Zhu, Y.; Xia, Y. Shape-Controlled Synthesis of Pd Nanocrystals in Aqueous Solutions. Adv. Funct. Mater. 2009, 19, 189–200. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhuang, J.; Wang, X. Single-phase aqueous approach toward Pd sub-10 nm nanocubes and Pd-Pt heterostructured ultrathin nanowires. Chem. Commun. 2009, 43, 6613–6615. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Ohtaki, M.; Matsubara, T.; Cao, M.T.; Nogami, M. New Experimental Evidences of Pt-Pd Bimetallic Nanoparticles with Core–shell Configuration and Highly Fine-Ordered Nanostructures by High-Resolution Electron Transmission Microscopy. J. Phys. Chem. C 2012, 116, 12265–12274. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.; Deng, K.; Gui, L.; Tang, Y. Preparation of Tractable Platinum, Rhodium, and Ruthenium Nanoclusters with Small Particle Size in Organic Media. Chem. Mater. 2000, 12, 1622–1627. [Google Scholar] [CrossRef]
- Wei, T.; Fan, Z.; Luo, G.; Zheng, C.; Xie, D. A rapid and efficient method to prepare exfoliated graphite by microwave irradiation. Carbon 2009, 47, 337–339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Videla, A.H.M.; Ban, S.; Specchia, S.; Zhang, L.; Zhang, J. Non-noble Fe-Nx electrocatalysts supported on the reduced graphene oxide for oxygen reduction reaction. Carbon 2014, 76, 386–400. [Google Scholar] [CrossRef]
- Ohma, A.; Shinohara, K.; Iiyama, A.; Yoshida, T.; Daimaru, A. Membrane and Catalyst Performance Targets for Automotive Fuel Cells by FCCJ Membrane, Catalyst, MEA WG. ECS Trans. 2011, 41, 775–784. [Google Scholar]
- Qin, W.; Han, L.; Bi, H.; Jian, J.; Wu, X.; Gao, P. Hydrogen storage in a chemical bond stabilized Co9S8-graphene layered structure. Nanoscale 2015, 7, 20180–20187. [Google Scholar] [CrossRef]
- Xing, R.; Wang, W.; Jiao, T.; Ma, K.; Zhang, Q.; Hong, W.; Qiu, H.; Zhou, J.; Zhang, L.; Peng, Q. Bioinspired polydopamine sheathed nanofibers containing carboxylate graphene oxide nanosheet for high-efficient dyes scavenger. ACS Sustain. Chem. Eng. 2017, 5, 4948–4956. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Guo, W.; Chen, T.; Wang, H.; Yu, S.; Gao, F. Highly Oxidized Graphene Anchored Ni(OH)2 Nanoflakes as Pseudocapacitor Materials for Ultrahigh Loading Electrode with High Areal Specific Capacitance. J. Phys. Chem. C 2014, 118, 24866–24876. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, H.; Zhong, H.; Ma, Y. A facile synthesis of Pd/C cathode electrocatalyst for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2011, 36, 725–731. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, H.; Zhong, H.; Xu, T.; Jin, H. Carbon-supported Pd-Pt cathode electrocatalysts for proton exchange membrane fuel cells. J. Power Sources 2011, 196, 3523–3529. [Google Scholar] [CrossRef]
- Li, C.; Sato, R.; Kanehara, M.; Zeng, H.; Bando, Y.; Teranishi, T. Controllable Polyol Synthesis of Uniform Palladium Icosahedra: Effect of Twinned Structure on Deformation of Crystalline Lattices. Angew. Chem. Int. Ed. 2009, 121, 6883–6887. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.D. Experimental studies of small particle structures. Rep. Prog. Phys. 1994, 57, 603–649. [Google Scholar] [CrossRef]
- Li, H.; Sun, G.; Li, N.; Sun, S.; Su, D.; Xin, Q. Design and Preparation of Highly Active Pt-Pd/C Catalyst for the Oxygen Reduction Reaction. J. Phys. Chem. C 2007, 111, 5605–5617. [Google Scholar] [CrossRef]
- Czerwiński, A.; Grdeń, M.; Łukaszewski, M. Dual mechanism of hydrogen desorption from palladium alloys postulated on the basis of cyclic voltammetric studies. J. Solid State Eletrochem. 2004, 8, 411–415. [Google Scholar] [CrossRef]
- Duncan, H.; Lasia, A. Mechanism of hydrogen adsorption/absorption at thin Pd layers on Au (1 1 1). Electrochim. Acta 2007, 52, 6195–6205. [Google Scholar] [CrossRef]
- Żurowski, A.; Grdeń, M.; Łukaszewski, M. Electrosorption of hydrogen into palladium-rhodium alloys. Electrochim. Acta 2006, 51, 3112–3117. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Kuśmierczyk, K.; Kotowski, J.; Siwek, H.; Czerwiński, A. Electrosorption of hydrogen into palladium-gold alloys. J. Solid State Electrochem. 2003, 7, 69–76. [Google Scholar] [CrossRef]
- Grdeń, M.; Piaścik, A.; Koczorowski, Z.; Czerwiński, A. Hydrogen electrosorption in Pd-Pt alloys. J. Electroanal. Chem. 2002, 532, 35–42. [Google Scholar] [CrossRef]
- Grdeń, M.; Łukaszewski, M.; Jerkiewiczb, G.; Czerwiński, A. Electrochemical behaviour of palladium electrode: Oxidation, electrodissolution and ionic adsorption. Electrochim. Acta 2008, 53, 7583–7598. [Google Scholar] [CrossRef]
- Savadogo, O.; Lee, K.; Oishi, K.; Mitsushima, S.; Kamiya, N.; Ota, K.I. New palladium alloys catalyst for the oxygen reduction reaction in an acid medium. Electrochem. Commun. 2004, 6, 105–109. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Chen, T.; Guo, W. Surfactant-Free Synthesis of Reduced Graphene Oxide Supported Well-Defined Polyhedral Pd-Pt Nanocrystals for Oxygen Reduction Reaction. Catalysts 2019, 9, 756. https://doi.org/10.3390/catal9090756
Tang Y, Chen T, Guo W. Surfactant-Free Synthesis of Reduced Graphene Oxide Supported Well-Defined Polyhedral Pd-Pt Nanocrystals for Oxygen Reduction Reaction. Catalysts. 2019; 9(9):756. https://doi.org/10.3390/catal9090756
Chicago/Turabian StyleTang, Yongfu, Teng Chen, and Wenfeng Guo. 2019. "Surfactant-Free Synthesis of Reduced Graphene Oxide Supported Well-Defined Polyhedral Pd-Pt Nanocrystals for Oxygen Reduction Reaction" Catalysts 9, no. 9: 756. https://doi.org/10.3390/catal9090756