Selective Conversion of Glucose to 5-Hydroxymethylfurfural by Using L-Type Zeolites with Different Morphologies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
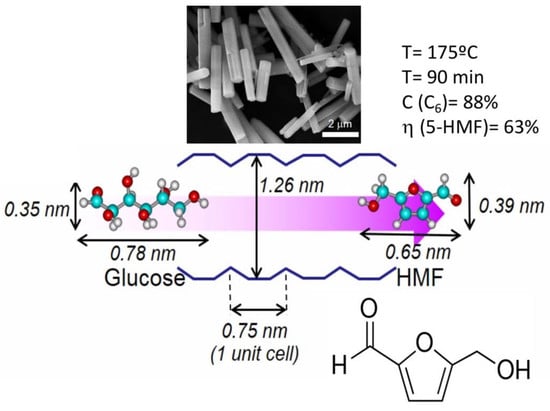
2.2. Catalytic Study
3. Materials and Methods
3.1. Chemicals and Mterials
3.2. Synthesis of L-Type Zeolites
3.3. Characterization of LTL Zeolites
3.4. Catalytic Reaction
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab.: Golden, CO, USA, 2004; Volume 1. [Google Scholar]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.D.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C 6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Delidovich, I.; Leonhard, K.; Palkovits, R. Cellulose and hemicellulose valorisation: An integrated challenge of catalysis and reaction engineering. Energy Environ. Sci. 2014, 7, 2803. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A.; Corma Canos, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Tang, X.; Xu, J.; Wu, Z.; Lin, L.; Liu, S. Selective transformation of 5-hydroxymethylfurfural into the liquid fuel 2,5-dimethylfuran over carbon-supported ruthenium. Ind. Eng. Chem. Res. 2014, 53, 3056–3064. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Rackemann, D.W.; Doherty, W.O. The conversion of lignocellulosics to levulinic acid. Biofuels Bioprod. Bioref. 2011, 5, 198–214. [Google Scholar] [CrossRef] [Green Version]
- Nikolla, E.; Roman-Leshkov, Y.; Moliner, M.; Davis, M.E. “One-Pot” Synthesis of 5-(Hydroxymethyl)furfural from Carbohydrates using Tin-Beta Zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef] [Green Version]
- Yabushita, M.; Kobayashi, H.; Fukuoka, A. Catalytic transformation of cellulose into platform chemicals. Appl. Catal. B Environ. 2014, 145, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Climent, M.J.; Corma, A.; Iborra, S. Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts. Green Chem. 2011, 13, 520–540. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A review. Catal. Today 2014, 234, 42–58. [Google Scholar]
- García-Sancho, C.; Fúnez-Núñez, I.; Moreno-Tost, R.; Santamaría-González, J.; Pérez-Inestrosa, E.; Fierro, J.L.G.; Maireles-Torres, P. Beneficial effects of calcium chloride on glucose dehydration to 5-hydroxymethylfurfural in the presence of alumina as catalyst. Appl. Catal. B Environ. 2017, 206, 617–625. [Google Scholar] [CrossRef]
- Moreno-Recio, M.; Santamaría-González, J.; Maireles-Torres, P. Brönsted and Lewis acid ZSM-5 zeolites for the catalytic dehydration of glucose into 5-hydroxymethylfurfural. Chem. Eng. J. 2016, 303, 22–30. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Moreno-Recio, M.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Production of 5-hydroxymethylfurfural from glucose using aluminium doped MCM-41 silica as acid catalyst. Appl. Catal. B Environ. 2015, 164, 70–76. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Moreno-Recio, M.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Mesoporous tantalum oxide as catalyst for dehydration of glucose to 5-hydroxymethylfurfural. Appl. Catal. B Environ. 2014, 154, 190–196. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.W.; Abu-Omar, M.M. Conversion of carbohydrates and lignocellulosic biomass into 5-hydroxymethylfurfural using AlCl3 center dot 6H(2)O catalyst in a biphasic solvent system. Green Chem. 2012, 14, 509–513. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Dumesic, J.A. Solvent effects on fructose dehydration to 5-hydroxymethylfurfural in biphasic systems saturated with inorganic salts. Top. Catal. 2009, 52, 297–303. [Google Scholar] [CrossRef]
- Teychené, J.; Roux-De Balman, H.; Galier, S. Role of the triple solute/ion/water interactions on the saccharide hydration: A volumetric approach. Carbohydr. Res. 2017, 448, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Combs, E.; Cinlar, B.; Pagan-Torres, Y.; Dumesic, J.A.; Shanks, B.H. Influence of alkali and alkaline earth metal salts on glucose conversion to 5-hydroxymethylfurfural in an aqueous system. Catal. Commun. 2013, 30, 1–4. [Google Scholar] [CrossRef]
- Moreno-Recio, M.; Jiménez-Morales, I.; Arias, P.L.; Santamaría-González, J.; Maireles-Torres, P. The Key Role of Textural Properties of Aluminosilicates in the Acid-Catalysed Dehydration of Glucose into 5-Hydroxymethylfurfural. ChemistrySelect 2017, 2, 2444–2451. [Google Scholar] [CrossRef]
- Xu, S.; Pan, D.; Hu, F.; Wu, Y.; Wang, H.; Chen, Y.; Yuan, H.; Gao, L.; Xiao, G. Highly efficient Cr/β-zeolite catalyst for conversion of carbohydrates into 5-hydroxymethylfurfural: Characterization and performance. Fuel Process. Technol. 2019, 190, 38–46. [Google Scholar] [CrossRef]
- Huang, Y.J.; Qi, G.R.; Chen, L.S. Effects of morphology and composition on catalytic performance of double metal cyanide complex catalyst. Appl. Catal. A Gen. 2003, 240, 263–271. [Google Scholar] [CrossRef]
- Remy, M.J.; Stanica, D.; Poncelet, G.; Feijen, E.J.P.; Grobet, P.J.; Martens, J.A.; Jacobs, P.A. Dealuminated H-Y zeolites: Relation between physicochemical properties and catalytic activity in heptane and decane isomerization. J. Phys. Chem. 1996, 100, 12440–12447. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bombem, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Ng, E.P.; Awala, H.; Tan, K.H.; Adam, F.; Retoux, R.; Mintova, S. EMT-type zeolite nanocrystals synthesized from rice husk. Microporous Mesoporous Mater. 2015, 204, 204–209. [Google Scholar] [CrossRef]
- Valtchev, V.P.; Bozhilov, K.N. Transmission electron microscopy study of the formation of FAU-type zeolite at room temperature. J. Phys. Chem. B 2004, 108, 15587–15598. [Google Scholar] [CrossRef]
- Xu, B.; Bordiga, S.; Prins, R.; van Bokhoven, J.A. Effect of framework Si/Al ratio and extra-framework aluminum on the catalytic activity of Y zeolite. Appl. Catal. A Gen. 2007, 333, 245–253. [Google Scholar] [CrossRef]
- Mu, M.; Harvey, G.; Prins, R. Quantitative multinuclear MAS NMR studies of zeolites. Microporous Mesoporous Mater. 2000, 34, 281–290. [Google Scholar]
- Chen, F.R.; Davis, J.G.; Fripiat, J.J. Aluminum coordination and Lewis acidity in transition aluminas. J. Catal. 1992, 133, 263–278. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Zeolite catalyst design for the conversion of glucose to furans and other renewable fuels. Fuel 2019, 258, 115851. [Google Scholar] [CrossRef]
- Tang, Z.; Su, J. Direct conversion of cellulose to 5-hydroxymethylfurfural (HMF) using an efficient and inexpensive boehmite catalyst. Carbohydr. Res. 2019, 481, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2015, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Fúnez-Núñez, I.; García-Sancho, C.; Cecilia, J.A.; Moreno-Tost, R.; Pérez-Inestrosa, E.; Serrano-Cantador, L.; Maireles-Torres, P. Synergistic effect between CaCl2 and γ-Al2O3 for furfural production by dehydration of hemicellulosic carbohydrates. Appl. Catal. A Gen. 2019, 585, 117188. [Google Scholar] [CrossRef]
- Choorefe, M.Y.; Juan, J.C.; Oi, L.E.; Ling, T.C.; Ng, E.P.; Noorsaadah, A.R.; Centi, G.; Lee, K.T. The role of nanosized zeolite y in the H2 -free catalytic deoxygenation of triolein. Catal. Sci. Technol. 2019, 9, 772–782. [Google Scholar]














| Binding Energy (eV) | Si/Al Molar Ratio * | K/Al Molar Ratio * | ||||
|---|---|---|---|---|---|---|
| Si 2p | Al 2p | O 1s | K 2p3/2 | |||
| NEEDLE-LTL | 102.8 | 74.3 | 532.1 | 293.3 | 4.23 (3.00) | 0.45 (0.63) |
| ROD-LTL | 102.9 | 74.5 | 532.2 | 293.4 | 4.54 (2.90) | 0.45 (0.55) |
| CYL-LTL | 103.2 | 74.6 | 532.4 | 293.7 | 4.43 (3.45) | 1.28 (1.07) |
| SLangmuir (m2 g−1) | Smicro (m2 g−1) | Vp (cm3 g−1) | Length (μm) | Width (μm) | Total Acidity (μmol m−2) * | |
|---|---|---|---|---|---|---|
| NEEDLE-LTL | 248 | 235 | 0.100 | 4.46 | 0.63 | 6.73 |
| ROD-LTL | 303 | 293 | 0.120 | 1.43 | 1.38 | 6.17 |
| CYL-LTL | 219 | 199 | 0.090 | 3.70 | 0.97 | 4.88 |
| Py-FTIR Acidity (µmol/g) | B/L | |||||||
|---|---|---|---|---|---|---|---|---|
| Lewis (L) | Brönsted (B) | Total (L + B) | ||||||
| 150 °C | 300 °C | 150 °C | 300 °C | 150 °C | 300 °C | 150 °C | 300 °C | |
| ROD-LTL | 89.7 | 24.40 | 101.1 | 84.4 | 152.23 | 84.51 | 3.52 | - |
| CYL-LTL | 126.08 | 32.08 | 128.07 | 152.85 | 254.15 | 184.93 | 1.02 | 4.76 |
| NEEDLE-LTL | 120.55 | 31.76 | 161.39 | 154.50 | 281.95 | 186.26 | 1.34 | 4.86 |
| Molar Composition | Aging (h) | Synthesis Temperature (°C) | Synthesis Time (h) | ||||
|---|---|---|---|---|---|---|---|
| K2O | Al2O3 | SiO2 | H2O | ||||
| ROD-LTL | 10.0 | 1 | 20 | 800 | 18 | 180 | 72 |
| NEEDLE-LTL | 10.2 | 1 | 20 | 1100 | 18 | 180 | 72 |
| CYL-LTL | 10.2 | 1 | 20 | 1030 | 18 | 180 | 72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginés-Molina, M.J.; Ahmad, N.H.; Mérida-Morales, S.; García-Sancho, C.; Mintova, S.; Eng-Poh, N.; Maireles-Torres, P. Selective Conversion of Glucose to 5-Hydroxymethylfurfural by Using L-Type Zeolites with Different Morphologies. Catalysts 2019, 9, 1073. https://doi.org/10.3390/catal9121073
Ginés-Molina MJ, Ahmad NH, Mérida-Morales S, García-Sancho C, Mintova S, Eng-Poh N, Maireles-Torres P. Selective Conversion of Glucose to 5-Hydroxymethylfurfural by Using L-Type Zeolites with Different Morphologies. Catalysts. 2019; 9(12):1073. https://doi.org/10.3390/catal9121073
Chicago/Turabian StyleGinés-Molina, María José, Nur Hidayahni Ahmad, Sandra Mérida-Morales, Cristina García-Sancho, Svetlana Mintova, Ng Eng-Poh, and Pedro Maireles-Torres. 2019. "Selective Conversion of Glucose to 5-Hydroxymethylfurfural by Using L-Type Zeolites with Different Morphologies" Catalysts 9, no. 12: 1073. https://doi.org/10.3390/catal9121073