Core/Shell Structure of Mesoporous Carbon Spheres and g-C3N4 for Acid Red 18 Decolorization
Abstract
:1. Introduction
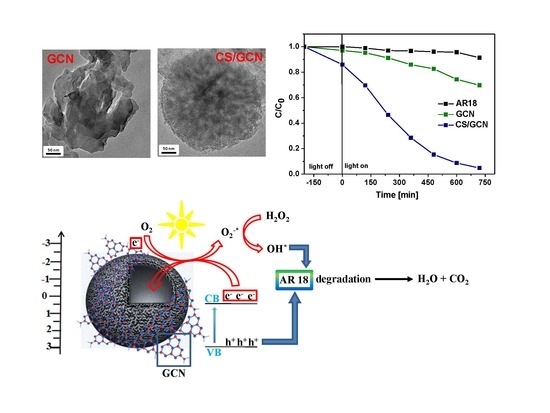
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Materials
3.1.1. Preparation of Graphitic Carbon Nitride (GCN)
3.1.2. Preparation of Mesoporous Carbon Spheres (CS)
3.1.3. Preparation of CS/GCN Core/Shell Hybrid
3.2. Photocatalytic Tests
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, Z.; Sun, Y. Dong. Graphitic carbon nitride based nanocomposites: a review. Nanoscale 2015, 7, 15–37. [Google Scholar] [PubMed]
- Capilli, G.; Costamagna, M.; Sordello, F.; Minero, C. Synthesis, characterization and photocatalytic performance of p-type carbon nitride. Appl. Catal. B 2019, 242, 121–131. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Cao, D.; Wang, Y.; Zhu, Y. Peroxymonosulfate enhanced visible light photocatalytic degradation bisphenol A by single-atom dispersed Ag mesoporous g-C3N4 hybrid. Appl. Catal. B 2017, 211, 79–88. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, J.; Jiang, C.; Cheng, B.; Yu, J. First principle investigation of halogen-doped monolayer g-C3N4 photocatalyst. Appl. Catal. B 2017, 207, 27–34. [Google Scholar] [CrossRef]
- Hao, R.; Wang, G.; Tang, H.; Sun, L.; Xu, C.; Han, D. Template-free preparation of macro/mesoporous g-C3N4/TiO2 heterojunction photocatalysts with enhanced visible light photocatalytic activity. Appl. Catal. B 2016, 187, 47–58. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Chen, D.; Wang, K.; Hong, W.; Zong, R.; Yao, W.; Zhu, Y. Visible light photoactivity enhancement via CuTCPP hybridized g-C3N4 nanocomposite. Appl. Catal. B 2015, 166–167, 366–373. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A review. Appl. Catal. B 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Jun, Y.S.; Hong, W.H.; Antonietti, M.; Thomas, A. Mesoporous, 2D Hexagonal Carbon Nitride and Titanium Nitride/Carbon Composites. Adv. Mater. 2009, 21, 4270–4274. [Google Scholar] [CrossRef]
- Yang, S.B.; Feng, X.L.; Wang, X.C.; Mullen, K. Graphene-Based Carbon Nitride Nanosheets as Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reactions. Angew. Chem. Int. Ed. 2011, 50, 5339–5343. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Z.; Gao, X.; Yao, W.; Wei, W.; Chen, X.; Zong, R.; Zhu, Y. Core-shell g-C3N4@ZnO composites as photoanodes with double synergistic effects for enhanced visible-light photoelectrocatalytic activities. Appl. Catal. B 2017, 217, 169–180. [Google Scholar] [CrossRef]
- Karimi, L. Combination of mesoporous titanium dioxide with MoS2 nanosheets for high photocatalytic activity. Pol. J. Chem. Technol. 2017, 19, 56–60. [Google Scholar] [CrossRef]
- Xu, M.; Han, L.; Dong, S. Facile Fabrication of Highly Efficient g-C3N4/Ag2O Heterostructured Photocatalysts with Enhanced Visible-Light Photocatalytic Activity. ACS Appl. Mater. Interf. 2013, 5, 12533–12540. [Google Scholar] [CrossRef]
- Xu, H.; Yan, J.; Xu, Y.; Song, Y.; Li, H.; Xia, J.; Huang, C.; Wan, H. Novel visible-light-driven AgX/graphite-like C3N4 (X = Br, I) hybrid materials with synergistic photocatalytic activity. Appl. Catal. B 2013, 129, 182–193. [Google Scholar] [CrossRef]
- Kumar, T.; Surendar, A.; Baruah, V.; Shanker, J. Synthesis of a novel and stable g-C3N4–Ag3PO4 hybrid nanocomposite photocatalyst and study of the photocatalytic activity under visible light irradiation. Mater. Chem. A 2013, 1, 5333–5340. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, L.; Zhu, J.; Chen, M.; Shi, W.; Xie, J. Novel p–n heterojunction photocatalyst constructed by porous graphite-like C3N4 and nanostructured BiOI: facile synthesis and enhanced photocatalytic activity. Dalton Trans. 2013, 42, 15726–15734. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Xiao, X.; Guo, L. Synthesis and characterization of composite visible light active photocatalysts MoS2–g-C3N4 with enhanced hydrogen evolution activity. Int. J. HydrogenEnergy 2013, 38, 6960–6969. [Google Scholar] [CrossRef]
- Cao, S.W.; Yuan, Y.P.; Fang, J.; Shahjamali, M.M.; Boey, F.Y.; Barber, J.; Loo, S.C.; Xue, C. In-situ growth of CdS quantum dots on g-C3N4 nanosheets for highly efficient photocatalytic hydrogen generation under visible light irradiation. Int. J. Hydrogen Energy 2013, 38, 1258–1266. [Google Scholar] [CrossRef]
- Xu, H.; Song, Y.; Song, Y.; Zhu, J.; Zhu, T.; Liu, C.; Zhao, D.; Zhang, Q.; Li, H. Synthesis and characterization of g-C3N4/Ag2CO3 with enhanced visible-light photocatalytic activity for the degradation of organic pollutants. RSC Adv. 2014, 4, 34539–34547. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A. Magnetically separable ternary g-C3N4/Fe3O4/BiOI nanocomposites: Novel visible-light-driven photocatalysts based on graphitic carbon nitride. J. Colloid Interface Sci. 2016, 465, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Baruah, A.; Tonda, S.; Kumar, B.; Shanker, V.; Sreedhar, B. Cost-effective and eco-friendly synthesis of novel and stable N-doped ZnO/g-C3N4 core–shell nanoplates with excellent visible-light responsive photocatalysis. Nanoscale 2014, 6, 4830–4842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, M.; Cui, W.; Sui, H. Synthesis and characterization of a core–shell BiVO4@g-C3N4 photo-catalyst with enhanced photocatalytic activity under visible light irradiation. RSC Adv. 2017, 7, 8167–8177. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, X.; Duan, L.; Liu, R.; Li, H. Enhanced visible light photocatalytic activity in SnO2@g-C3N4 core-shell structures. Mater. Sci. Eng. B 2017, 218, 23–30. [Google Scholar] [CrossRef]
- Lu, D.; Wang, H.; Zhao, X.; Kondamareddy, K.K.; Ding, J.; Li, C.; Fang, P. Highly Efficient Visible-Light-Induced Photoactivity of Z-Scheme g-C3N4/Ag/MoS2 Ternary Photocatalysts for Organic Pollutant Degradation and Production of Hydrogen. ACS Sustain. Chem. Eng. 2017, 5, 1436–1445. [Google Scholar] [CrossRef]
- Ma, L.; Wanga, G.; Jiangb, C.; Bao, H.; Xu, Q. Synthesis of core-shell TiO2@g-C3N4 hollow microspheres for efficient photocatalytic degradation of rhodamine B under visible light. Appl. Surf. Sci. 2017, 430, 263–272. [Google Scholar] [CrossRef]
- Yao, Y.; Lu, F.; Zhu, Y.; Wei, F.; Liu, X.; Lian, C.; Wang, S. Magnetic core–shell CuFe2O4@C3N4 hybrids for visible light photocatalysis of Orange II. J. Hazard. Mater. 2015, 297, 224–233. [Google Scholar] [CrossRef]
- Liu, S.; Ke, J.; Sun, H.; Liu, J.; Tade, M.O.; Wang, S. Size dependence of uniformed carbon spheres in promoting graphitic carbon nitride toward enhanced photocatalysis. Appl. Catal. B 2017, 204, 358–364. [Google Scholar] [CrossRef]
- Li, K.; Xie, X.; Zhang, W.D. Photocatalysts based on g-C3N4-encapsulating carbon spheres with high visible light activity for photocatalytic hydrogen evolution. Carbon 2016, 110, 356–366. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopouloa, T.; Ioannidis, N.; Boukosa, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs thermal exfoliation of g-C3N4 for NOx removal under visible light irradiation. Appl. Catal. B 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lin, L.; Wang, B.; Wang, X. Graphitic carbon nitride polymers toward sustainable photoredox catalysis. Angew.Chem. Int. Ed. 2015, 54, 12868–12884. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Fan, H.; Fu, K.; Lei, S.; Hu, Q. Protonation of Graphitic Carbon Nitride (g-C3N4) for an Electrostatically Self-Assembling Carbon@g-C3N4 Core–Shell Nanostructure toward High Hydrogen Evolution. ACS Sustain. Chem. Eng. 2017, 5, 7093–7103. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Surface charge modification via protonation of graphitic carbon nitride (g-C3N4) for electrostatic self-assembly construction of 2D/2D reduced graphene oxide (rGO)/g-C3N4 nanostructures toward enhanced photocatalytic reduction of carbon dioxide to methane. NanoEnergy 2015, 13, 757–770. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, D.; Yan, J.; Jia, X.; Li, Y.; Yu, P.; Zhang, T.J. The pore size distribution and its relationship with shale gas capacity in organic-rich mudstone of Wufeng-Longmaxi Formations, Sichuan Basin, China. Nat. Gas Geosci. 2016, 1, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Jiang, Z.; Xu, C.; Liu, B.; Liu, G.; Wang, P.; Li, X.; Chen, W.; Ning, C.; Wang, Z. Effect of pore structure on shale oil accumulation in the lower third member of the Shahejie formation, Zhanhua Sag, eastern China: Evidence from gas adsorption and nuclear magnetic resonance. Mar. Pet. Geol. 2017, 88, 932–949. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, Z.; Chen, L.; Yin, L.; Li, Z.; Zhang, C.; Tang, X.; Wang, G. Pore structure characterization for the Longmaxi and Niutitang shales in the Upper Yangtze Platform, South China: Evidence from focused ion beam–He ion microscopy, nano-computerized tomography and gas adsorption analysis. Mar.Pet. Geol. 2018, 77, 1323–1337. [Google Scholar] [CrossRef]
- Bommier, C.; Luo, W.; Gao, W.-Y.; Greaney, A.; Ma, S.; Ji, X. Predicting capacity of hard carbon anodes in sodium-ion batteries using porosity measurements. Carbon 2014, 76, 165–174. [Google Scholar] [CrossRef]
- Mohini, R.; Lakshminarasimhan, N. Coupled semiconductor nanocomposite g-C3N4/TiO2 with enhanced visible light photocatalytic activity. Mater. Res. Bull. 2016, 76, 370–375. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Wu, F.; Ni, Y.; Kokot, S. Preparation of protonated, two-dimensional graphitic carbon nitride nanosheets by exfoliation, and their application as a fluorescent probe for trace analysis of copper(II). Microchim. Acta 2016, 183, 773–780. [Google Scholar] [CrossRef]
- Pawar, R.C.; Kang, S.; Park, J.H.; Kim, J.H.; Ahn, S.; Lee, C.S. Room-temperature synthesis of nanoporous 1D microrods of graphitic carbon nitride (gC3N4) with highly enhanced photocatalytic activity and stability. Sci. Rep. 2016, 6, 31147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bai, X.; Liu, D.; Wang, J.; Zhu, Y. Enhanced catalytic activity of potassium-doped graphitic carbon nitride induced by lower valence position. Appl. Catal. B 2015, 164, 77–81. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yang, W.J.; Chen, X.J.; Wang, J.; Zhu, Y.F. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 layer. Appl. Catal. B 2018, 220, 337–347. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Kandula, S.; Shanker, V.J. Fe-doped and -mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J. Mater. Chem. A 2014, 2, 6772–6780. [Google Scholar] [CrossRef]
- Hu, S.W.; Yang, L.W.; Tian, Y.; Wei, X.L.; Ding, J.W.; Zhong, J.X.; Chu, P.K. Simultaneous nanostructure and heterojunction engineering of graphitic carbon nitride via in situ Ag doping for enhanced photoelectrochemical activity. Appl. Catal. B 2015, 163, 611–622. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.G.; Trapalis, C.J. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Zinin, P.V.; Ming, L.C.; Sharma, S.K.; Khabashesku, V.N.; Liu, X.; Hong, S.; Endo, S.; Acosta, T. Ultraviolet and near-infrared Raman spectroscopy of graphitic C3N4 phase. Chem. Phys. Lett. 2009, 472, 69–73. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Gao, C.; Hsu, W.K.; Zhu, Y.; Huczko, A.; Bystrzejewski, M.; Roe, M.; Lee, C.Y.; Acquah, S.; Kroto, H.; et al. Large-scale synthesis and characterization of carbon spheres prepared by direct pyrolysis of hydrocarbons. Carbon 2005, 43, 1944–1953. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric Graphitic Carbon Nitride as a Heterogeneous Organocatalyst: From Photochemistry to Multipurpose Catalysis to Sustainable Chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, S.; Wu, Z.; Zhao, S.; Piao, L. Enhanced photocatalytic activity towards degradation and H2 evolution over one dimensional TiO2@MWCNTs heterojunction. Appl. Surf. Sci. 2017, 402, 360–368. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.Z.; Wu, Y.; Zeng, G.M.; Chen, X.H.; Leng, L.J.; Li, H. Synthesis and applications of novel graphitic carbon nitride/metal-organic frameworks mesoporous photocatalyst for dyes removal. Appl. Catal. B 2015, 174, 445–454. [Google Scholar] [CrossRef]
- Ye, S.; Qiu, L.-G.; Yuan, Y.-P.; Zhu, Y.-J.; Xia, J.; Zhu, J.-F. Facile fabrication of magnetically separable graphitic carbon nitride photocatalysts with enhanced photocatalytic activity under visible light. J. Mater. Chem. A 2013, 1, 3008–3015. [Google Scholar] [CrossRef]
- Cinelli, G.; Cuomo, F.; Ambrosone, L.; Colella, M.; Ceglie, A.; Venditti, F.; Lopez, F.J. Photocatalytic degradation of a model textile dye using carbon-doped titanium dioxide and visible light. Water Process Eng. 2017, 20, 71–77. [Google Scholar] [CrossRef]
- Venditti, F.; Cuomo, F.; Ceglie, A.; Avino, P.; Russo, M.V.; Lopez, F. Visible Light caffeic acid degradation by carbon-doped titanium dioxide. Langmuir 2015, 31, 3627–3634. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Lee, K.T.; Mohamed, A.R. Preparation and photocatalytic properties of visible light-driven samarium-doped ZnO nanorods. Ceram. Int. 2013, 39, 5833–5843. [Google Scholar] [CrossRef]
- Raza, W.; Haque, M.M.; Muneer, M.; Fleisch, M.; Hakki, A.; Bahnemann, D.J. Photocatalytic degradation of different chromophoric dyes in aqueous phase using La and Mo doped TiO2 hybrid carbon spheres. Alloys Compd. 2015, 632, 837–844. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Liu, W.; Zhang, S. Highly enhanced photocatalytic degradation of methylene blue over the indirect all-solid-state Z-scheme g-C3N4-RGO-TiO2 nanoheterojunctions. Appl. Surf. Sci. 2017, 405, 60–70. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Wang, S.; Yao, F.; Sun, J.; Wang, Y.; Zhang, C.; Li, X.; Niu, C.; Wang, D.; et al. Graphene oxide and carbon nitride nanosheets co-modified silver chromate nanoparticles with enhanced visible-light photoactivity and anti-photocorrosion properties towards multiple refractory pollutants degradation. Appl. Catal. B 2017, 209, 493–505. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Lu, X.; Fan, J.; Cai, X.; Gao, B.; Miao, R.; Wang, J.; Lv, Y. Comparative study of the photocatalytic performance for the degradation of different dyes by ZnIn2S4: adsorption, active species, and pathways. RSC Adv. 2017, 7, 12292–12300. [Google Scholar] [CrossRef] [Green Version]
- Konstantinou, K.I.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: A review. Appl. Catal. B 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Osugi, M.E.; Chanmanee, W.; Chenthamarakshan, C.R.; Zanoni, M.V.B.; Kajitichyanukal, P.; Krishnan-Ayer, R. Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J.Photochem. Photobiol. C 2008, 9, 171–192. [Google Scholar] [CrossRef]











| Sample | Brunauer−Emmett−Teller (BET)Surface Area (m2/g) | Total Pore Volume (cm3/g) | Band Gap Energy (eV) |
|---|---|---|---|
| CS | 290.34 | 0.23 | - |
| GCN | 17.82 | 0.13 | 2.72 |
| CS/GCN | 92.09 | 0.21 | 2.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baca, M.; Aleksandrzak, M.; Mijowska, E.; Kaleńczuk, R.J.; Zielińska, B. Core/Shell Structure of Mesoporous Carbon Spheres and g-C3N4 for Acid Red 18 Decolorization. Catalysts 2019, 9, 1007. https://doi.org/10.3390/catal9121007
Baca M, Aleksandrzak M, Mijowska E, Kaleńczuk RJ, Zielińska B. Core/Shell Structure of Mesoporous Carbon Spheres and g-C3N4 for Acid Red 18 Decolorization. Catalysts. 2019; 9(12):1007. https://doi.org/10.3390/catal9121007
Chicago/Turabian StyleBaca, Martyna, Małgorzata Aleksandrzak, Ewa Mijowska, Ryszard J. Kaleńczuk, and Beata Zielińska. 2019. "Core/Shell Structure of Mesoporous Carbon Spheres and g-C3N4 for Acid Red 18 Decolorization" Catalysts 9, no. 12: 1007. https://doi.org/10.3390/catal9121007