In-Situ Arc Discharge-Derived FeSn2/Onion-Like Carbon Nanocapsules as Improved Stannide-Based Electrocatalytic Anode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of FeSn2/OLC Nanocapsules
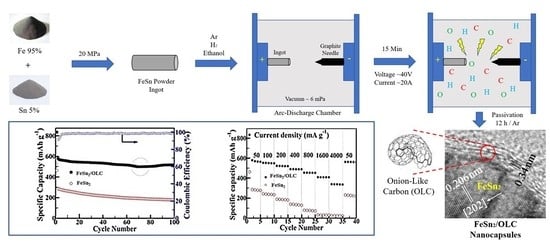
2.2. Proposed Formation Mechanism of FeSn2/OLC Nanocapsules
2.3. Electrochemical Performance of LIB Cells
3. Materials and Methods
3.1. Materials
3.2. Synthesis of FeSn2/OLC Nanocapsules
3.3. Physicochemical Characterizations of FeSn2/OLC Nanocapsules
3.4. Preparation of FeSn2/OLC Nanocapsules as Stannide-Based Electrocatalytic Anodes and Fabrication of Their LIB Cells
3.5. Electrochemical Characterizations of LIB Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, R.; Wang, J.G.; Liu, H.; Liu, H.; Jin, D.; Liu, X.; Shen, C.; Xie, K.; Wei, B. Coaxial MoS2@Carbon hybrid fibers: A low-cost anode material for high-performance Li-ion batteries. Materals 2017, 10, 174. [Google Scholar] [CrossRef]
- Liu, C.J.; Xue, F.H.; Huang, H.; Yu, X.H.; Xie, C.J.; Shi, M.S.; Cao, G.Z.; Jung, Y.G.; Dong, X.L. Preparation and Electrochemical properties of Fe-Sn (C) Nanocomposites as Anode for Lithium-ion Batteries. Electrochim. Acta 2014, 129, 93–99. [Google Scholar] [CrossRef]
- Diouf, B.; Pode, R. Potential of lithium-ion batteries in renewable energy. Renew. Energy 2015, 76, 375–380. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Chen, J. Recent Progress in Advanced Materials for Lithium Ion Batteries. Materals 2013, 6, 156–183. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, J.; Liu, C.; Ji, S.; Zhou, Y. Facile Synthesis of FeSn2 Alloy Nanoparticles as Anode Materials for Lithium-Ion Batteries. Energy Environ. Focus 2013, 2, 63–67. [Google Scholar] [CrossRef]
- Leibowitz, J.; Allcorn, E.; Manthiram, A. FeSn2-TiC nanocomposite alloy anodes for lithium ion batteries. J. Power Sources 2015, 295, 125–130. [Google Scholar] [CrossRef]
- Qin, J.; Liu, D.; Zhang, X.; Zhao, N.; Shi, C.; Liu, E.; He, F.; Ma, L.; Li, Q.; Li, J.; et al. One-step synthesis of SnCo nanoconfined in hierarchical carbon nanostructures for lithium ion battery anode. Nanoscale 2017, 9, 15856–15864. [Google Scholar] [CrossRef]
- Lee, J.M.; Chang, W.S.; Yu, B.C.; Kim, H.; Im, D.; Doo, S.G.; Sohn, H.J. Enhancement of cyclability using recombination reaction of Cu for Sn2Fe nanocomposite anode for lithium-ion batteries. Electrochem. Commun. 2010, 12, 928–932. [Google Scholar] [CrossRef]
- Liu, X.; Or, S.W.; Jin, C.; Lv, Y.; Feng, C.; Sun, Y. NiO/C nanocapsules with onion-like carbon shell as anode material for lithium ion batteries. Carbon 2013, 60, 215–220. [Google Scholar] [CrossRef]
- Han, D.; Song, G.; Liu, B.; Yan, H. Core-shell-structured nickel ferrite/onion-like carbon nanocapsules: An anode material with enhanced electrochemical performance for lithium-ion batteries. RSC Adv. 2015, 5, 42875–42880. [Google Scholar] [CrossRef]
- Kravchyk, K.; Protesescu, L.; Bodnarchuk, M.I.; Krumeich, F.; Yarema, M.; Walter, M.; Guntlin, C.; Kovalenko, M.V. Monodisperse and inorganically capped Sn and Sn/SnO2nanocrystals for high-performance Li-ion battery anodes. J. Am. Chem. Soc. 2013, 135, 4199–4202. [Google Scholar] [CrossRef]
- Li, Q.-Y.; Pan, Q.-C.; Yang, G.-H.; Lin, X.-L.; Yan, Z.-X.; Wang, H.-Q.; Huang, Y.-G. Synthesis of Sn/MoS2/C composites as high-performance anodes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 20375–20381. [Google Scholar] [CrossRef]
- Ferrara, G.; Damen, L.; Arbizzani, C.; Inguanta, R.; Piazza, S.; Sunseri, C.; Mastragostino, M. SnCo nanowire array as negative electrode for lithium-ion batteries. J. Power Sources 2011, 196, 1469–1473. [Google Scholar] [CrossRef]
- Chamas, M.; Lippens, P.E.; Jumas, J.C.; Hassoun, J.; Panero, S.; Scrosati, B. Electrochemical impedance characterization of FeSn2electrodes for Li-ion batteries. Electrochim. Acta 2011, 56, 6732–6736. [Google Scholar] [CrossRef]
- Armbrüster, M.; Schnelle, W.; Cardoso-Gil, R.; Grin, Y. Chemical bonding in compounds of the CuAl2family: MnSn2, FeSn2and CoSn2. Chem. A Eur. J. 2010, 16, 10357–10365. [Google Scholar] [CrossRef]
- Han, D.; Or, S.W.; Dong, X.; Liu, B. FeSn2/defective onion-like carbon core-shell structured nanocapsules for high-frequency microwave absorption. J. Alloys Compd. 2017, 695, 2605–2611. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.L.; Simon, P. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef]
- Zeiger, M.; Jäckel, N.; Mochalin, V.N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172–3196. [Google Scholar] [CrossRef]
- Van Aken, K.L.; Maleski, K.; Mathis, T.S.; Breslin, J.P.; Gogotsi, Y. Processing of Onion-like Carbon for Electrochemical Capacitors. ECS J. Solid State Sci. Technol. 2017, 6, M3103–M3108. [Google Scholar] [CrossRef]
- Wang, S.; He, M.; Walter, M.; Krumeich, F.; Kravchyk, K.V.; Kovalenko, M.V. Monodisperse CoSn2and FeSn2nanocrystals as high-performance anode materials for lithium-ion batteries. Nanoscale 2018, 10, 6827–6831. [Google Scholar] [CrossRef]
- Armbruster, M.; Schmidt, M.; Cardoso-Gil, R.; Borrmann, H.; Grin, Y. Crystal structures of iron distannide, FeSn2, and cobalt distannide, CoSn2. Z. fur Krist.-New Cryst. Struct. 2007, 222, 83–84. [Google Scholar] [CrossRef]
- Nwokeke, U.G.; Alcántara, R.; Tirado, J.L.; Stoyanova, R.; Yoncheva, M.; Zhecheva, E. Electron paramagnetic resonance, X-ray diffraction, mössbauer spectroscopy, and electrochemical studies on nanocrystalline FeSn2obtained by reduction of salts in tetraethylene glycol. Chem. Mater. 2010, 22, 2268–2275. [Google Scholar] [CrossRef]
- Liu, X.; Wu, N.; Cui, C.; Zhou, P.; Sun, Y. Enhanced rate capability and cycling stability of core/shell structured CoFe2O4/onion-like C nanocapsules for lithium-ion battery anodes. J. Alloys Compd. 2015, 644, 59–65. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Z.; Geng, D.; Lin, Y.; Wang, Y.; An, J.; He, J.; Li, D.; Liu, W.; Zhang, Z. Structure and electromagnetic properties of both regular and defective onion-like carbon nanoparticles. Carbon 2015, 95, 910–918. [Google Scholar] [CrossRef]
- Lei, J.P.; Dong, X.L.; Zhu, X.G.; Lei, M.K.; Huang, H.; Zhang, X.F.; Lu, B.; Park, W.J.; Chung, H.S. Formation and characterization of intermetallic Fe-Sn nanoparticles synthesized by an arc discharge method. Intermetallics 2007, 15, 1589–1594. [Google Scholar] [CrossRef]
- Thompson, C.V. On the role of diffusion in phase selection during reactions at interfaces. J. Mater. Res. 1992, 7, 367–373. [Google Scholar] [CrossRef]
- Growth Mechanisms of Carbon Onions Obtained by Thermal Treatment - Diamond Films. Texas Powerful Smart. 2019. Available online: https://www.texaspowerfulsmart.com/diamond-films/growth-mechanisms-of-carbon-onions-obtained-by-thermal-treatment.html (accessed on 7 November 2019).
- Zhang, C.Q.; Tu, J.P.; Huang, X.H.; Yuan, Y.F.; Wang, S.F.; Mao, F. Preparation an electrochemical performances of nanoscale FeSn2as anode material for lithium ion batteries. J. Alloys Compd. 2008, 457, 81–85. [Google Scholar] [CrossRef]
- Yuan, Y.; Amine, K.; Lu, J.; Shahbazian-Yassar, R. Understanding materials challenges for rechargeable ion batteries with in situ transmission electron microscopy. Nat. Commun. 2017, 8, 15806. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, D.Y. Enhancement of capacitance by electrochemical oxidation of nanodiamond derived carbon nano-onions. Electrochim. Acta 2014, 139, 82–87. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wu, Y.; Li, X.; Yu, J.; Sun, Y. FeS@onion-like carbon nanocapsules embedded in amorphous carbon for the lithium ion batteries with excellent cycling stability. Ceram. Int. 2018, 44, 13654–13661. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Aoki, Y.; Komaba, S. Understanding Particle-Size-Dependent Electrochemical Properties of Li2MnO3-Based Positive Electrode Materials for Rechargeable Lithium Batteries. J. Phys. Chem. C 2016, 120, 875–885. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Xu, Y.; Huang, L.; Li, J.; Sun, S.; Zhao, J. Superiority of the bi-phasic mixture of a tin-based alloy nanocomposite as the anode for lithium ion batteries. J. Mater. Chem. A 2015, 3, 3794–3800. [Google Scholar] [CrossRef]
- Osaka, T.; Mukoyama, D.; Nara, H. Review—Development of Diagnostic Process for Commercially Available Batteries, Especially Lithium Ion Battery, by Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2015, 162, A2529–A2537. [Google Scholar] [CrossRef]
- Qin, F.; Zhang, K.; Fang, J.; Lai, Y.; Li, Q.; Zhang, Z.; Li, J. High performance lithium sulfur batteries with a cassava-derived carbon sheet as a polysulfides inhibitor. New J. Chem. 2014, 38, 4549–4554. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Choi, P.P.; Kim, J.S.; Kwon, D.H.; Gerasimov, K.B. Decomposition of intermetallics during high-energy ball-milling. Mater. Sci. Eng. A 2007, 449–451, 1083–1086. [Google Scholar] [CrossRef]
- Chaitoglou, S.; Sanaee, M.R.; Aguiló-Aguayo, N.; Bertran, E. Arc-discharge synthesis of iron encapsulated in carbon nanoparticles for biomedical applications. J. Nanomater. 2014, 2014, 178524. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Chatterjee, A.; Man, L.H.; Or, S.W. In-Situ Arc Discharge-Derived FeSn2/Onion-Like Carbon Nanocapsules as Improved Stannide-Based Electrocatalytic Anode Materials for Lithium-Ion Batteries. Catalysts 2019, 9, 950. https://doi.org/10.3390/catal9110950
Han D, Chatterjee A, Man LH, Or SW. In-Situ Arc Discharge-Derived FeSn2/Onion-Like Carbon Nanocapsules as Improved Stannide-Based Electrocatalytic Anode Materials for Lithium-Ion Batteries. Catalysts. 2019; 9(11):950. https://doi.org/10.3390/catal9110950
Chicago/Turabian StyleHan, Dandan, Amrita Chatterjee, Long Hin Man, and Siu Wing Or. 2019. "In-Situ Arc Discharge-Derived FeSn2/Onion-Like Carbon Nanocapsules as Improved Stannide-Based Electrocatalytic Anode Materials for Lithium-Ion Batteries" Catalysts 9, no. 11: 950. https://doi.org/10.3390/catal9110950