Effects of Additives and Metals on Crystallization of Nano-Sized HZSM-5 Zeolite for Glycerol Aromatization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Additives
2.1.1. Characterization of the n-HZSM-5 Catalysts
2.1.2. Catalytic Performance of the n-HZSM-5 Catalysts
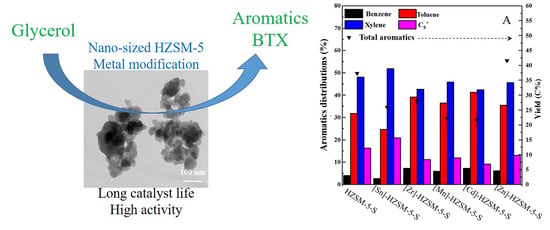
2.2. Effects of the Metals
2.2.1. Characterization of the Modified n-HZSM-5 Catalysts
2.2.2. Catalytic Performance of Modified [M]-HZSM-5-S
3. Materials and Methods
3.1. Materials
3.2. Catalysts Preparation and Characterization
3.3. Catalytic Reaction Procedure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ardi, M.S.; Aroua, M.K.; Hashim, N.A. Progress, prospect and challenges in glycerol purification process: A review. Renew. Sust. Energ. Rev. 2015, 42, 1164–1173. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; Cesar, A.D. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sust. Energ. Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiao, G.M.; Varma, A. A universal procedure for crude glycerol purification from different feedstocks in biodiesel production: experimental and simulation study. Ind. Eng. Chem. Res. 2013, 52, 14291–14296. [Google Scholar] [CrossRef]
- Rekha, V.; Raju, N.; Sumana, C.; Lingaiah, N. Continuous hydrogenolysis of glycerol to 1,2-propanediol over Bi-metallic Ni-Ag supported on gamma-Al2O3 Catalysts. Catal. Lett. 2017, 147, 1441–1452. [Google Scholar] [CrossRef]
- Viswanadham, B.; Nagaraju, N.; Rohitha, C.N.; Vishwanathan, V.; Chary, K.V.R. Synthesis, characterization and catalytic dehydration of glycerol to acrolein over phosphotungstic acid supported Y-zeolite catalysts. Catal. Lett. 2018, 148, 397–406. [Google Scholar] [CrossRef]
- Xiao, W.Y.; Wang, F.; Xiao, G.M. Performance of hierarchical HZSM-5 zeolites prepared by NaOH treatments in the aromatization of glycerol. RSC Adv. 2015, 5, 63697–63704. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, M.X.; Yang, X.H.; Gao, L.J.; Xiao, G.M. The effect of hierarchical pore architecture on one-step catalytic aromatization of glycerol: Reaction routes and catalytic performances. Mol. Catal. 2017, 432, 144–154. [Google Scholar] [CrossRef]
- Jang, H.S.; Bae, K.; Shin, M.; Kim, S.M.; Kim, C.U.; Suh, Y.W. Aromatization of glycerol/alcohol mixtures over zeolite H-ZSM-5. Fuel 2014, 134, 439–447. [Google Scholar] [CrossRef]
- Tamiyakul, S.; Ubolcharoen, W.; Tungasmita, D.N.; Jongpatiwut, S. Conversion of glycerol to aromatic hydrocarbons over Zn-promoted HZSM-5 catalysts. Catal. Today 2015, 256, 325–335. [Google Scholar] [CrossRef]
- Hoang, T.Q.; Zhu, X.L.; Sooknoi, T.; Resasco, D.E.; Mallinson, R.G. A comparison of the reactivities of propanal and propylene on HZSM-5. J. Catal. 2010, 271, 201–208. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, Z.P.; Chen, P.; Ruan, R. Microwave-assisted catalytic fast pyrolysis of biomass for bio-oil production using chemical vapor deposition modified HZSM-5 catalyst. Bioresour. Technol. 2015, 197, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, M.; Lopez-Sanchez, J.A.; He, Q.; Morgan, D.J.; Ryabenkova, Y.; Bartley, J.K.; Carley, A.F.; Taylor, S.H.; Kiely, C.J.; Khalid, K.; et al. Modified zeolite ZSM-5 for the methanol to aromatics reaction. Catal. Sci. Technol. 2012, 2, 105–112. [Google Scholar] [CrossRef]
- Hoang, T.Q.; Zhu, X.L.; Danuthai, T.; Lobban, L.L.; Resasco, D.E.; Mallinson, R.G. Conversion of glycerol to alkyl-aromatics over zeolites. Energy Fuels 2010, 24, 3804–3809. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Bai, T.; Chen, T.F.; Fan, W.T.; Zhang, X. Conversion of methanol to light aromatics on Zn-modified nano-HZSM-5 zeolite catalysts. Ind. Eng. Chem. Res. 2014, 53, 14932–14940. [Google Scholar] [CrossRef]
- Wang, F.; Kang, X.; Zhou, M.X.; Yang, X.H.; Gao, L.J.; Xiao, G.M. Sn and Zn modified HZSM-5 for one-step catalytic upgrading of glycerol to value-added aromatics: Synergistic combination of impregnated Sn particles, ALD introduced ZnO film and HZSM-5 zeolite. Appl. Catal. A-Gen. 2017, 539, 80–89. [Google Scholar] [CrossRef]
- Austin, D.; Wang, A.G.; He, P.; Qian, H.; Zeng, H.B.; Song, H. Catalytic valorization of biomass derived glycerol under methane: Effect of catalyst synthesis method. Fuel 2018, 216, 218–226. [Google Scholar] [CrossRef]
- Qi, R.Y.; Fu, T.J.; Wan, W.L.; Li, Z. Pore fabrication of nano-ZSM-5 zeolite by internal desilication and its influence on the methanol to hydrocarbon reaction. Fuel. Process. Technol. 2017, 155, 191–199. [Google Scholar] [CrossRef]
- Wan, Z.J.; Wu, W.; Li, G.; Wang, C.F.; Yang, H.; Zhang, D.K. Effect of SiO2/Al2O3 ratio on the performance of nanocrystal ZSM-5 zeolite catalysts in methanol to gasoline conversion. Appl. Catal. A-Gen. 2016, 523, 312–320. [Google Scholar] [CrossRef]
- Wang, F.; Chu, X.Z.; Zhu, F.X.; Li, Q.Q.; Wu, F.Y.; Liu, B.H. Study on catalytic performance and deactivation behavior of HZSM-5 in aromatization of glycerol. Energy Technol. 2018, 6, 2238–2246. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.Q.; Zhao, J.C.; Liu, C.G. Hierarchical porous ZSM-5 zeolite synthesized by in situ zeolitization and its coke deposition resistance in aromatization reaction. Chin. J. Chem. 2012, 30, 597–603. [Google Scholar] [CrossRef]
- Chu, S.; Guo, X.C.; Li, J.; Bai, J.; Mu, X.D.; Yang, L.N. Synthesis of Ga2O3/HZSM-5@cubic ordered mesoporous SiO2 with template Pluronic F127 to improve its catalytic performance in the aromatization of methanol. J. Porous Mater. 2017, 24, 1069–1078. [Google Scholar] [CrossRef]
- Groen, J.C.; Jansen, J.C.; Moulijn, J.A.; Perez-Ramirez, J. Optimal aluminum-assisted mesoporosity development in MFI zeolites by desilication. J. Phys. Chem. B 2004, 108, 13062–13065. [Google Scholar] [CrossRef]
- Hoang, T.Q.; Zhu, X.L.; Lobban, L.L.; Resasco, D.E.; Mallinson, R.G. Effects of HZSM-5 crystallite size on stability and alkyl-aromatics product distribution from conversion of propanal. Catal. Commun. 2010, 11, 977–981. [Google Scholar] [CrossRef]
- Jia, Y.M.; Wang, J.W.; Zhang, K.; Feng, W.; Liu, S.B.; Ding, C.M.; Liu, P. Nanocrystallite self-assembled hierarchical ZSM-5 zeolite microsphere for methanol to aromatics. Microporous Mesoporous Mater. 2017, 247, 103–115. [Google Scholar] [CrossRef]
- Yang, L.Z.; Liu, Z.Y.; Liu, Z.; Peng, W.Y.; Liu, Y.Q.; Liu, C.G. Correlation between H-ZSM-5 crystal size and catalytic performance in the methanol-to-aromatics reaction. Chin. J. Catal. 2017, 38, 683–690. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.X.; He, N.; Liu, C.Y.; Guo, H.C. A comparison on the hydrothermal stability of nano-sized H-ZSM-5 zeolite modified by ammonium dihydrogen phosphate and trimethylphosphate. Catal. Lett. 2019, 149, 2169–2179. [Google Scholar] [CrossRef]
- Fu, T.J.; Chang, J.W.; Shao, J.A.; Li, Z. Fabrication of a nano-sized ZSM-5 zeolite with intercrystalline mesopores for conversion of methanol to gasoline. J. Energy Chem. 2017, 26, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.H.; Zhang, W.W.; Jia, S.Y.; Liu, Y.; Ran, J.Y.; Ren, H.T.; Hou, J.W. Novel pathway for the synthesis of monodisperse MCM-41 nanospheres with different particle size distributions. Mater. Lett. 2013, 98, 138–141. [Google Scholar] [CrossRef]
- Tian, H.F.; Zhang, Z.Z.; Chang, H.; Ma, X.X. Catalytic performance of imidazole modified HZSM-5 for methanol to aromatics reaction. J. Energy Chem. 2017, 26, 574–583. [Google Scholar] [CrossRef] [Green Version]
- Tao, H.X.; Yang, H.; Liu, X.H.; Ren, J.W.; Wang, Y.Q.; Lu, G.Z. Highly stable hierarchical ZSM-5 zeolite with intra- and inter-crystalline porous structures. Chem. Eng. J. 2013, 225, 686–694. [Google Scholar] [CrossRef]
- Demadis, K.D.; Mavredaki, E.; Somara, M. Additive-driven dissolution enhancement of colloidal Silica. 1. basic principles and relevance to water treatment. Ind. Eng. Chem. Res. 2011, 50, 12587–12595. [Google Scholar] [CrossRef]
- Yokoi, T.; Mochizuki, H.; Namba, S.; Kondo, J.N.; Tatsumi, T. Control of the Al distribution in the framework of ZSM-5 zeolite and its evaluation by solid-state NMR technique and catalytic properties. J. Phys. Chem. C 2015, 119, 15303–15315. [Google Scholar] [CrossRef]
- Erofeev, V.I.; Adyaeva, L.V.; Ryabova, N.V. Effect of high-temperature steam treatment of high-silica zeolites of the ZSM-5 type on their acidity and selectivity of formation of lower olefins from straight-run naphthas. Russ. J. Appl. Chem. 2003, 76, 95–98. [Google Scholar] [CrossRef]
- Pan, D.H.; Song, X.H.; Yang, X.H.; Gao, L.J.; Wei, R.P.; Zhang, J.; Xiao, G.M. Efficient and selective conversion of methanol to para-xylene over stable H [Zn,Al]ZSM-5/SiO2 composite catalyst. Appl. Catal. A-Gen. 2018, 557, 15–24. [Google Scholar] [CrossRef]
- Yang, X.H.; Wang, F.; Wei, R.P.; Li, S.; Wu, Y.F.; Shen, P.X.; Wang, H.Z.; Gao, L.J.; Xiao, G.M. Synergy effect between hierarchical structured and Sn-modified H[Sn, Al]ZSM-5 zeolites on the catalysts for glycerol aromatization. Microporous Mesoporous Mater. 2018, 257, 154–161. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, W.Y.; Gao, L.J.; Xiao, G.M. Enhanced performance of glycerol to aromatics over Sn-containing HZSM-5 zeolites. RSC Adv. 2016, 6, 42984–42993. [Google Scholar] [CrossRef]
- Veses, A.; Puertolas, B.; Lopez, J.M.; Callen, M.S.; Solsona, B.; Garcia, T. Promoting deoxygenation of bio-Oil by metal-loaded hierarchical ZSM-5 zeolites. ACS Sustain. Chem. Eng. 2016, 4, 1653–1660. [Google Scholar] [CrossRef]
- El Morsi, A.K.; Shokry, S.A. Transformation of n-octane into aromatic hydrocarbon over ZSM-5 zeolites. Pet. Sci. Technol. 2000, 18, 1195–1207. [Google Scholar] [CrossRef]
- Meng, Y.T.; Genuino, H.C.; Kuo, C.H.; Huang, H.; Chen, S.Y.; Zhang, L.C.; Rossi, A.; Suib, S.L. One-step hydrothermal synthesis of manganese-containing MFI-type zeolite, Mn-ZSM-5, characterization, and catalytic oxidation of hydrocarbons. J. Am. Chem. Soc. 2013, 135, 8594–8605. [Google Scholar] [CrossRef]
- Pan, D.H.; Xu, S.Q.; Miao, Y.N.; Xu, N.N.; Wang, H.Z.; Song, X.H.; Gao, L.J.; Xiao, G.M. A highly active and stable Zn@C/HZSM-5 catalyst using Zn@C derived from ZIF-8 as a template for conversion of glycerol to aromatics. Catal. Sci. Technol. 2019, 9, 739–752. [Google Scholar] [CrossRef]







| Samples | HZSM-5-C | HZSM-5-N | HZSM-5-S | HZSM-5-P | HZSM-5-K |
|---|---|---|---|---|---|
| Crystallinity a/% | 100.1 | 90.9 | 103.1 | 79.1 | 83.6 |
| Catalysts | SBET a (m2/g) | Smicro b (m2/g) | Vtotal (mL/g) | Vmicro b (mL/g) | Vmeso c (mL/g) |
|---|---|---|---|---|---|
| HZSM-5 | 375.5 | 207.5 | 0.49 | 0.11 | 0.38 |
| HZSM-5-C | 379.2 | 225.8 | 0.31 | 0.12 | 0.18 |
| HZSM-5-N | 385.8 | 202.4 | 0.53 | 0.11 | 0.39 |
| HZSM-5-S | 400.0 | 199.0 | 0.57 | 0.11 | 0.45 |
| HZSM-5-P | 408.4 | 216.1 | 0.67 | 0.11 | 0.55 |
| HZSM-5-K | 468.6 | 298.7 | 0.72 | 0.15 | 0.59 |
| Catalysts | Total Acid (mmol/g) | Weak Acid (mmol/g) | Strong Acid (mmol/g) | SiO2/Al2O3 (mol %) |
|---|---|---|---|---|
| HZSM-5 | 0.42 | 0.15 | 0.27 | 66.4 |
| HZSM-5-C | 0.86 | 0.34 | 0.52 | 54.9 |
| HZSM-5-N | 0.92 | 0.37 | 0.55 | 56.4 |
| HZSM-5-S | 1.24 | 0.44 | 0.80 | 50.3 |
| HZSM-5-P | 0.31 | 0.12 | 0.19 | 70.3 |
| HZSM-5-K | 0.52 | 0.19 | 0.33 | 63.7 |
| Catalysts | SBET a (m2/g) | Smicro b (m2/g) | Vmicro b (mL/g) | Vmeso c (mL/g) | Total Acid (mmol/g) | Weak Acid (mmol/g) | Strong Acid (mmol/g) |
|---|---|---|---|---|---|---|---|
| [Sn]-HZSM-5-S | 384.0 | 193.9 | 0.10 | 0.52 | 0.88 | 0.42 | 0.46 |
| [Zr]-HZSM-5-S | 376.6 | 203.1 | 0.11 | 0.46 | 1.02 | 0.41 | 0.61 |
| [Mn]-HZSM-5-S | 391.5 | 198.0 | 0.11 | 0.49 | 1.09 | 0.43 | 0.67 |
| [Cd]-HZSM-5-S | 386.2 | 205.4 | 0.11 | 0.61 | 1.11 | 0.42 | 0.69 |
| [Zn]-HZSM-5-S | 384.4 | 186.8 | 0.10 | 0.77 | 1.14 | 0.42 | 0.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Gao, L.; Xiao, G. Effects of Additives and Metals on Crystallization of Nano-Sized HZSM-5 Zeolite for Glycerol Aromatization. Catalysts 2019, 9, 899. https://doi.org/10.3390/catal9110899
Xu W, Gao L, Xiao G. Effects of Additives and Metals on Crystallization of Nano-Sized HZSM-5 Zeolite for Glycerol Aromatization. Catalysts. 2019; 9(11):899. https://doi.org/10.3390/catal9110899
Chicago/Turabian StyleXu, Wei, Lijing Gao, and Guomin Xiao. 2019. "Effects of Additives and Metals on Crystallization of Nano-Sized HZSM-5 Zeolite for Glycerol Aromatization" Catalysts 9, no. 11: 899. https://doi.org/10.3390/catal9110899