Construction of an Ultrasensitive and Highly Selective Nitrite Sensor Using Piroxicam-Derived Copper Oxide Nanostructures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of As-Prepared Px-CuO NSs
2.1.1. SEM Study
2.1.2. XRD Study
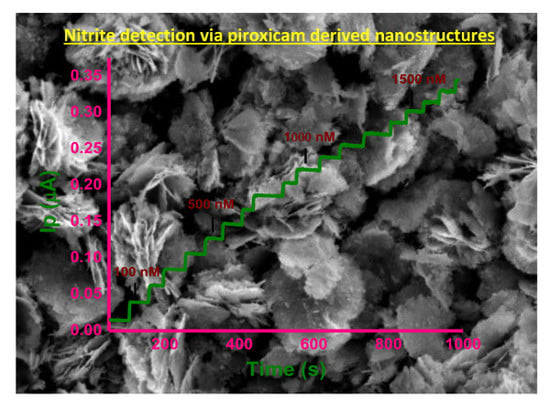
2.2. Sensing Studies
2.2.1. Sensitivity Investigation
2.2.2. Effect of Scan Rate
2.2.3. CV Calibration
2.2.4. Reproducibility and Long-Term Stability
2.3. Amperometric Study
2.3.1. Amperometric Calibration
2.3.2. Interference Effect
2.4. Figures of Merit
2.5. Application of the Developed Sensor for Nitrite Detection in Real Water Samples
3. Experimental Section
3.1. Chemical and Reagents
3.2. Synthesis of Piroxicam-Based CuO Nanostructures (Px-CuO NSs) via a Hydrothermal Method
3.3. Construction of the Px-CuO NSs Sensor and Its Application for Nitrite Detection
3.4. Application of the Sensor to Real Samples
3.5. Instruments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuralay, F.; Dumangöz, M.; Tunç, S. Polymer/carbon nanotubes coated graphite surfaces for highly sensitive nitrite detection. Talanta 2015, 144, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, S.; Huang, H.; Cao, A.; Li, M.; He, L. Highly sensitive and selective spectrofluorimetric determination of nitrite in food products with a novel fluorogenic probe. Food Control 2016, 63, 117–121. [Google Scholar] [CrossRef]
- Yang, J.H.; Yang, H.; Liu, S.; Mao, L. Microwave-assisted synthesis graphite-supported Pd nanoparticles for detection of nitrite. Sens. Actuators B-Chem. 2015, 220, 652–658. [Google Scholar] [CrossRef]
- Lijinsky, W.; Epstein, S.S. Nitrosamines as environmental carcinogens. Nature 1970, 225, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.M.; Kleinbongard, P.; Ringwood, L.; Hito, R.; Hunter, C.J.; Schechter, A.N.; Gladwin, M.T.; Dejam, A. The measurement of blood and plasma nitrite by chemiluminescence: Pitfalls and solutions. Free Radic. Biol. Med. 2016, 41, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Betta, F.D.; Vitali, L.; Fett, R.; Costa, A.C.O. Development and validation of a sub-minute capillary zone electrophoresis method for determination of nitrate and nitrite in baby foods. Talanta 2014, 122, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Niedzielski, P.; Kurzyca, I.; Siepak, J. A new tool for inorganic nitrogen speciation study: Simultaneous determination of ammonium ion, nitrite and nitrate by ion chromatography with post-column ammonium derivatization by Nessler reagent and diode-array detection in rain water samples. Anal. Chim. Acta 2006, 577, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Canbay, E.; Sahin, B.; Kıran, M.; Akyilmaz, E. MWCNT-cysteamine-nafion modified gold electrode based on myoglobin for determination of hydrogen peroxide and nitrite. Bioelectrochemistry 2015, 101, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, T.; Nafady, A.; Talpur, F.N.; Sirajuddin; Agheem, M.H.; Shah, M.R.; Sherazi, S.T.H.; Soomro, R.A.; Siddiqui, S. Tranexamic acid derived gold nanoparticles modified glassy carbon electrode as sensitive sensor for determination of nalbuphine. Sens. Actuators B-Chem. 2015, 211, 359–369. [Google Scholar] [CrossRef]
- Hassan, S.S.; Nafady, A.; Sirajuddin; Solangi, A.R.; Kalhoro, M.S.; Abro, M.I.; Sherazi, S.T.H. Ultra-trace level electrochemical sensor for methylene blue dye based on nafion stabilized ibuprofen derived gold nanoparticles. Sens. Actuators B-Chem. 2015, 208, 320–326. [Google Scholar] [CrossRef]
- Soomro, R.A.; Hallam, K.R.; Ibupoto, Z.H.; Tahira, A.; Sherazi, S.T.H.; Sirajuddin; Jawaid, S.; Willander, M. Glutaric acid assisted fabrication of CuO nanostructures and their application in development of highly sensitive electrochemical sensor system for carbamates. Electroanalysis 2016, 28, 1634–1640. [Google Scholar] [CrossRef]
- Arain, M.; Nafady, A.; Sirajuddin; Ibupoto, Z.H.; Sherazi, S.T.H.; Shaikh, T.; Khan, H.; Alsalme, A.; Niaz, A.; Willander, M. Simpler and highly sensitive enzyme-free sensing of urea via NiO nanostructures modified electrode. RSC Adv. 2016, 6, 39001–39006. [Google Scholar] [CrossRef]
- Choi, K.I.; Kim, H.R.; Kim, K.M.; Liu, D.; Cao, G.; Lee, J.H. C2H5OH sensing characteristics of various Co3O4 nanostructures prepared by solvothermal reaction. Sens. Actuators B-Chem. 2010, 146, 183–189. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Abu-Hani, A.F.S.; Mahmoud, S.T.; Haik, Y. Selective H2S sensor based on CuO nanoparticles embedded in organic membranes. Sens. Actuators B-Chem. 2016, 231, 593–600. [Google Scholar] [CrossRef]
- Reddy, S.; Swamy, B.E.K.; Jayadevappa, H. CuO nanoparticle sensor for the electrochemical determination of dopamine. Electrochim. Acta 2012, 61, 78–86. [Google Scholar] [CrossRef]
- Soomro, R.A.; Hallam, K.R.; Ibupoto, Z.H.; Tahira, A.; Sherazi, S.T.H.; Sirajuddin; Memon, S.S.; Willander, M. Amino acid assisted growth of CuO nanostructures and their potential application in electrochemical sensing of organophosphate pesticide. Electrochim. Acta 2016, 190, 972–979. [Google Scholar] [CrossRef]
- Hyeonjeong, H.; Hyojin, K.; Dojin, K. Fabrication and characterization of CuO nanoparticles/ZnO nanorods heterojunction structure for room temperature NO gas sensor application. J. Nanosci. Nanotechnol. 2016, 11, 11608–11612. [Google Scholar]
- Haldorai, Y.; Kim, J.Y.; Vilian, A.T.E.; Heo, N.S.; Huh, Y.S.; Han, Y.K. An enzyme-free electrochemical sensor based on reduced graphene oxide/Co3O4 nanospindle composite for sensitive detection of nitrite. Sens. Actuators B Chem. 2016, 227, 92–99. [Google Scholar] [CrossRef]
- Salimi, A.; Hallaj, R.; Mamkhezri, H.; Hosaini, S.M.T. Electrochemical properties and electrocatalytic activity of FAD immobilized onto cobalt oxide nanoparticles: Application to nitrite detection. J. Electroanal. Chem. 2008, 619–620, 31–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Wen, F.; Yuan, B.; Wang, H. Fe3O4 nanospheres on MoS2 nanoflake: Electrocatalysis and detection of Cr(VI) and nitrite. J. Electroanal. Chem. 2016, 761, 14–20. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Krishnamoorthy, K.; Sekar, C.; Wilson, J.; Kim, S.J. A highly sensitive electrochemical sensor for nitrite detection based on Fe2O3 nanoparticles decorated reduced graphene oxide nanosheets. Appl. Catal. B Environ. 2014, 148–149, 22–28. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, F.; Zhang, X.; Yang, L. Facile synthesis of flower like copper oxide and their application to hydrogen peroxide and nitrite sensing. Chem. Cent. J. 2011, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fotouhi, L.; Fatollahzadeh, M.; Heravi, M.M. Electrochemical Behavior and Voltammetric determination of sulfaguanidine at a glassy carbon electrode modified with a multi-walled carbon nanotube. Int. J. Electrochem. Sci. 2012, 7, 3919–3928. [Google Scholar]
- Soomro, R.A.; Nafady, A.; Sirajuddin; Memon, N.; Sherazi, T.H.; Kalwar, N.H. L-cysteine protected copper nanoparticles as colorimetric sensor for mercuric ions. Talanta 2014, 130, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Haldorai, Y.; Hwang, S.K.; Gopalan, A.I.; Huh, Y.S.; Han, Y.K.; Voit, W.; Anand, G.S.; Lee, K.P. Direct electrochemistry of cytochrome c immobilized on titanium nitride/multi-walled carbon nanotube composite for amperometric nitrite biosensor. Biosens. Bioelectron. 2016, 79, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, B.; Sheng, Q.; Zheng, J. Electrochemical sensor for sensitive determination of nitrite based on the CuS-MWCNT nanocomposites. J. Electroanal. Chem. 2016, 769, 118–123. [Google Scholar] [CrossRef]









| Material on Electrode | Linear Range (nM) | LOD (nM) | Reference |
|---|---|---|---|
| Poly(vinylferrocenium)/multi-walled carbon nanotubes | 1000–400,000 | 100 | [1] |
| Graphite-supported Pd nanoparticles | 300–50,700 | 71 | [3] |
| Reduced graphene oxide/Co3O4 nanospindle | 1000–380,000 | 140 | [18] |
| Fe3O4 nanospheres on MoS2 nanoflake | 1000–2,630,000 | 500 | [20] |
| Hexamethylenetetramine-based flower-like CuO | 1000–91,500 | 360 | [22] |
| Cytochrome c immobilized on TiN nanoparticles-decorated multi-walled carbon nanotubes | 1000–2,000,000 | 1.4 | [25] |
| CuS supported on multiwall carbon nanotubes | 1000–8,100,000 | 330 | [26] |
| Px-CuO NSs | 100–1800 | 12 | This work |
| Sample Type | Nitrite Added (nM) | Nitrite Recovered (nM) 1 | Recovery (%) |
|---|---|---|---|
| Mineral water | 800 | 802.5 ± 3.1 | 100.3 |
| Tap water | 600 | 598.4 ± 1.9 | 99.7 |
| River water | 400 | 402.8 ± 2.4 | 100.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsalme, A.; Arain, M.; Nafady, A.; Sirajuddin. Construction of an Ultrasensitive and Highly Selective Nitrite Sensor Using Piroxicam-Derived Copper Oxide Nanostructures. Catalysts 2018, 8, 29. https://doi.org/10.3390/catal8010029
Alsalme A, Arain M, Nafady A, Sirajuddin. Construction of an Ultrasensitive and Highly Selective Nitrite Sensor Using Piroxicam-Derived Copper Oxide Nanostructures. Catalysts. 2018; 8(1):29. https://doi.org/10.3390/catal8010029
Chicago/Turabian StyleAlsalme, Ali, Munazza Arain, Ayman Nafady, and Sirajuddin. 2018. "Construction of an Ultrasensitive and Highly Selective Nitrite Sensor Using Piroxicam-Derived Copper Oxide Nanostructures" Catalysts 8, no. 1: 29. https://doi.org/10.3390/catal8010029