Solvent-Free Selective Condensations Based on the Formation of the Olefinic (C=C) Bond Catalyzed by Organocatalyst
Abstract
:1. Introduction
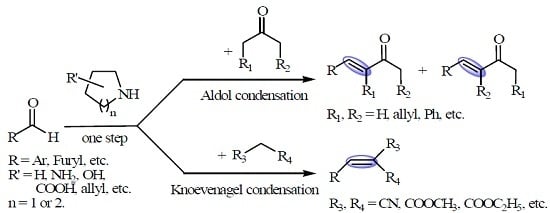
2. Results and Discussion
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sipos, G.Y.; Sirokman, F. Chalcon formation of different substituted acetophenones and p-hydroxy-benzaldehyde. Nature 1964, 202, 489. [Google Scholar] [CrossRef]
- Kozlov, N.G.; Basalaeva, L.I. Synthesis of unsymmetrical β-arylaminoketones. Russ. J. Gen. Chem. 2004, 74, 926–932. [Google Scholar] [CrossRef]
- Le Gall, E.; Texier-Boullet, F.; Hamelin, J. Simple access to α,β-unsaturated ketones by acid-catalyzed solvent-free reactions. Synth. Commun. 1999, 29, 3651–3657. [Google Scholar] [CrossRef]
- Li, J.T.; Chen, G.F.; Wang, J.X.; Li, T.S. Ultrasound promoted synthesis of α,α′-bis(substituted furfurylidene) cycloalkanones and chalcones. Synth. Commun. 1999, 29, 965–971. [Google Scholar] [CrossRef]
- Sivakumar, P.M.; Seenivasan, S.P.; Kumar, V.; Doble, M. Synthesis, antimycobacterial activity evaluation, and QSAR studies of chalcone derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Berkessel, A.; Groger, H. Asymmetric Organocatalysis; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- List, B.; Lerner, R.A.; Barbas, C.F. Proline-catalyzed direct asymmetric Aldol reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Notz, W.; List, B.J. Catalytic asymmetric synthesis of anti-1,2-Diols. Am. Chem. Soc. 2000, 122, 7386–7387. [Google Scholar] [CrossRef]
- List, B.; Pojarliev, P.; Castello, C. Proline-catalyzed asymmetric Aldol reactions between ketones and α-unsubstituted aldehydes. Org. Lett. 2001, 3, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, K.; Notz, W.; Bui, T.; Barbas, C.F. Amino acid catalyzed direct asymmetric aldol reactions: A bioorganic approach to catalytic asymmetric carbon-carbon bond-forming reactions. J. Am. Chem. Soc. 2001, 123, 5260–5267. [Google Scholar] [CrossRef] [PubMed]
- Co´rdova, A.; Notz, W.; Barbas, C.F., III. Proline-Catalyzed One-Step Asymmetric Synthesis of 5-Hydroxy-(2E)-hexenal from Acetaldehyde. J. Org. Chem. 2002, 67, 301–303. [Google Scholar] [CrossRef]
- Hajos, Z.G.; Parrish, D.R. Asymmetric synthesis of bicyclic intermediates of natural product chemistry. J. Org. Chem. 1974, 39, 1615–1621. [Google Scholar] [CrossRef]
- Eder, U.; Sauer, G.; Wiechert, R. New type of asymmetric cyclization to optically active steroid CD partial structures. Angew. Chem. Int. Ed. 1971, 10, 496–497. [Google Scholar] [CrossRef]
- Northrup, A.B.; MacMillan, D.W.C. The first direct and enantioselective cross-aldol reaction of aldehydes. J. Am. Chem. Soc. 2002, 124, 6798–6799. [Google Scholar] [CrossRef] [PubMed]
- Bøgevig, A.; Kumaragurubaran, N.; Jørgensen, K.A. Direct catalytic asymmetric aldol reactions of aldehydes. Chem. Commun. 2002, 620–621. [Google Scholar] [CrossRef]
- Dickerson, T.J.; Janda, K.D. Aqueous aldol catalysis by a nicotine metabolite. J. Am. Chem. Soc. 2002, 124, 3220–3221. [Google Scholar] [CrossRef] [PubMed]
- Cordova, A.; Notz, W.; Barbas, C.F., III. Direct organocatalytic aldol reactions in buffered aqueous media. Chem. Commun. 2002, 3024–3025. [Google Scholar] [CrossRef]
- Reymond, J.L.; Chen, Y.W. Catalytic, Enantioselective aldol reaction with an artificial aldolase assembled from a primary amine and an antibody. J. Org. Chem. 1995, 60, 6970–6979. [Google Scholar] [CrossRef]
- Dickerson, T.J.; Lovell, T.; Meijler, M.M.; Noodleman, L.; Janda, K.D. Nornicotine aqueous aldol reactions: Synthetic and theoretical investigations into the origins of catalysis. J. Org. Chem. 2004, 69, 6603–6609. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shang, Z.; Wu, T.; Fan, J.; Chen, X. Synthetic and theoretical study on proline-catalyzed Knoevenagel condensation in ionic liquid. J. Mol. Catal. A Chem. 2006, 253, 212–221. [Google Scholar] [CrossRef]
- Forbes, D.C.; Law, A.M.; Morrison, D.W. The Knoevenagel reaction: Analysis and recycling of the ionic liquid medium. Tetrahedron Lett. 2006, 47, 1699–1703. [Google Scholar] [CrossRef]
- Santamarta, F.; Verda, P.; Tojo, E. A simple, efficient and green procedure for Knoevenagel reaction in [MMIm][MSO4] ionic liquid. Catal. Commun. 2008, 9, 1779–1781. [Google Scholar] [CrossRef]
- Chimni, S.S.; Mahajan, D. Electron deficiency of aldehydes controls the pyrrolidine catalyzed direct cross-aldol reaction of aromatic/heterocyclic aldehydes and ketones in water. Tetrahedron 2005, 61, 5019–5025. [Google Scholar] [CrossRef]
- Bertelsen, S.; Marigo, M.; Brandes, S.; Diner, P.; Joergensen, K.A. Dienamine Catalysis: Organocatalytic Asymmetric γ-Amination of γ,β-Unsaturated Aldehydes. J. Am. Chem. Soc. 2006, 128, 12973–12980. [Google Scholar] [CrossRef] [PubMed]
- Heckel, T.; Konieczna, D.D.; Wilhelm, P. An Ionic Liquid Solution of Chitosan as Organocatalyst. Catalysts 2013, 3, 914–921. [Google Scholar] [CrossRef]


| Entry a | Catalysts | Time/h | Furaldehyde Con./% | Sele./% | |||
|---|---|---|---|---|---|---|---|
| 1a | 2a | 3a | 1a + 2a | ||||
| 1 |  | 1 h | 99.5 | 88.9 | 3.8 | 1.1 | 92.7 |
| 2 |  | 1 h | 94.6 | 87.2 | 0.5 | 3.2 | 87.7 |
| 3 |  | 1 h | 80.2 | 62.2 | 0.0 | 4.2 | 62.2 |
| 4 |  | 1 h | 26.3 | 62.5 | 0.8 | 7.5 | 63.3 |
| 20 h | 97.8 | 65.9 | 4.5 | 12.9 | 70.4 | ||
| 5 |  | 20 h | 29.2 | 4.6 | 0 | 73.0 | 4.6 |
| 6 |  | 20 h | 42.4 | 60.0 | 6.7 | 13.3 | 66.7 |
| 7 |  | 20 h | 37.1 | 13.9 | 11.1 | 8.3 | 25.0 |
| Entry a | Amount of Catalyst/mol % | Butanone/Furaldehyde/mol Ratio | Temp./°C | Time/h | Furaldehyde Con./% | Sele./% | |||
|---|---|---|---|---|---|---|---|---|---|
| 1a | 2a | 3a | 1a + 2a | ||||||
| 1 | 1 | 6:1 | 60 | 2 | 44.9 | 84.9 | 1.4 | 4.1 | 86.3 |
| 2 | 2 | 6:1 | 60 | 2 | 81.6 | 79.5 | 2.4 | 4.7 | 81.9 |
| 3 | 4 | 6:1 | 60 | 2 | 99.8 | 85.0 | 2.0 | 3.8 | 87.0 |
| 4 | 5 | 6:1 | 60 | 2 | 99.7 | 82.1 | 3.7 | 5.2 | 85.8 |
| 5 | 4 | 2:1 | 60 | 2 | 50.8 | 79.4 | 0.0 | 7.5 | 79.4 |
| 6 | 4 | 3:1 | 60 | 2 | 74.9 | 78.8 | 0.0 | 7.2 | 78.8 |
| 7 | 4 | 4:1 | 60 | 2 | 94.0 | 78.7 | 2.2 | 6.0 | 80.9 |
| 8 | 4 | 8:1 | 60 | 2 | 100 | 85.7 | 3.4 | 3.4 | 89.1 |
| 9 | 4 | 6:1 | 40 | 2 | 85.3 | 80.2 | 3.1 | 5.2 | 83.3 |
| 10 | 4 | 6:1 | 60 | 0.5 | 90.9 | 81.8 | 2.3 | 4.5 | 84.1 |
| 11 | 4 | 6:1 | 60 | 1 | 99.5 | 88.9 | 3.8 | 1.1 | 92.7 |
| Entry a | Ketone | Aldehyde | Reaction Conditions | Aldehyde Con./% | Sele./% | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 + 2 | |||||
| 1 |  |  | 60 °C/1 h | 99.4 | 51.4 | 39.2 | 51.4 | ||
| 2 |  |  | 60 °C/1 h | 90.4 | 90.9 | 1.4 | 90.9 | ||
| 3 |  |  | 60 °C/1 h | 56.3 | 100 | 0 | 0 | 0 | 100 |
| 60 °C/20 h | 86.2 | 100 | 0 | 0 | 0 | 100 | |||
| 4 |  |  | 40 °C/1 h | 100 | 81.7 | 15.0 | 1.3 | 0.4 | 96.7 |
| 5 |  |  | 40 °C/1 h | 98.8 | 83.1 | 13.1 | 1.2 | 0.5 | 96.2 |
| 6 |  |  | 40 °C/1 h | 98.6 | 80.6 | 15.4 | 1.2 | 0.6 | 96.0 |
| 7 |  |  | 40 °C/1 h | 95.7 | 39.7 | 47.2 | 5.2 | 4.7 | 86.9 |
| Entry a | Aldehyde | Methylene-Activated Substrates | Time | Product Yield/% |
|---|---|---|---|---|
| 1 b |  |  | 10 min | 94.5 |
| 2 b |  |  | 10 min | 92.5 |
| 3 |  |  | 1 h | 96.8 |
| 4 |  |  | 2 h | 91.8 |
| 5 |  |  | 0.5 h | 98.6 |
| 6 |  |  | 1 h | 90.2 |
| 7 |  |  | 2 h | 91.7 |
| 8 |  |  | 2 h | 69.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Jin, R.; Jin, F.; Kang, M.; Li, Z.; Chen, J. Solvent-Free Selective Condensations Based on the Formation of the Olefinic (C=C) Bond Catalyzed by Organocatalyst. Catalysts 2016, 6, 106. https://doi.org/10.3390/catal6070106
Song H, Jin R, Jin F, Kang M, Li Z, Chen J. Solvent-Free Selective Condensations Based on the Formation of the Olefinic (C=C) Bond Catalyzed by Organocatalyst. Catalysts. 2016; 6(7):106. https://doi.org/10.3390/catal6070106
Chicago/Turabian StyleSong, Heyuan, Ronghua Jin, Fuxiang Jin, Meirong Kang, Zhen Li, and Jing Chen. 2016. "Solvent-Free Selective Condensations Based on the Formation of the Olefinic (C=C) Bond Catalyzed by Organocatalyst" Catalysts 6, no. 7: 106. https://doi.org/10.3390/catal6070106