Regenerable Subnanometer Pd Clusters on Zirconia for Highly Selective Hydrogenation of Biomass-Derived Succinic Acid in Water
Abstract
:1. Introduction
2. Results and Discussion
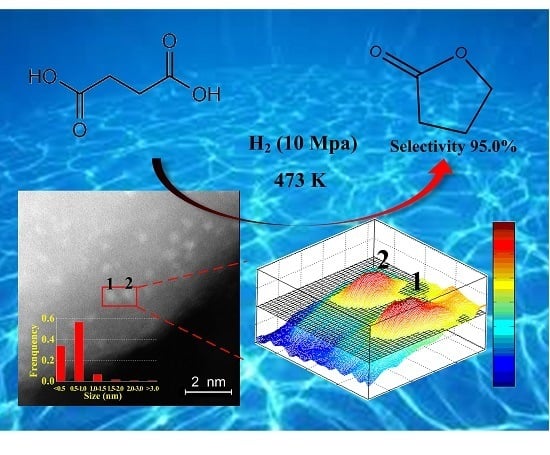
2.1. Electron Microscopy
2.2. Catalytic Activity of Pd/ZrO2
2.3. Stability of Pd/ZrO2 Catalysts
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Izarova, N.V.; Pope, M.T.; Kortz, U. Noble metals in polyoxometalates. Angew. Chem. Int. Ed. 2012, 51, 9492–9510. [Google Scholar] [CrossRef] [PubMed]
- Gholami, R.; Alyani, M.; Smith, K.J. Deactivation of Pd catalysts by water during low temperature methane oxidation relevant to natural gas vehicle converters. Catalysts 2015, 5, 561–594. [Google Scholar] [CrossRef]
- Manabe, K.; Yamaguchi, M. Catalyst-controlled site-selectivity switching in Pd-catalyzed cross-coupling of dihaloarenes. Catalysts 2014, 4, 307–320. [Google Scholar] [CrossRef]
- Huctching, G.S.; Zhang, Y.; Li, J.; Yonemoto, B.T.; Zhou, X.G.; Zhu, K.K.; Jiao, F. In situ formation of cobalt oxide nanocubanes as efficient oxygen. J. Am. Chem. Soc. 2015, 137, 4223–4229. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Kovarik, L.; Szanyi, J. CO2 reduction on supported Ru/Al2O3 catalysts: Cluster size dependence of product selectivity. ACS Catal. 2013, 3, 2449–2455. [Google Scholar] [CrossRef]
- Lu, H.; Li, W.C.; Hou, Z.; Schuth, S.F. Molecular level dispersed Pd clusters in the carbon walls of ordered mesoporous carbon as a highly selective alcohol oxidation catalyst. Chem. Commun. 2007, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Uzun, A.; Ortalan, V.; Browning, N.D.; Gates, B.C. A site-isolated mononuclear iridium complex catalyst supported on MgO: Characterization by spectroscopy and aberration-corrected scanning transmission electron microscopy. J. Catal. 2010, 269, 318–328. [Google Scholar] [CrossRef]
- Yan, H.; Cheng, H.; Yi, H.; Lin, Y.; Yao, T.; Wang, C.; Li, J.; Wei, S.; Lu, J. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: Remarkable performance in selective hydrogenation of 1, 3-butadiene. J. Am. Chem. Soc. 2015, 137, 10484–10487. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.J.; Delariva, A.T.; Lin, S.; Johnson, R.S.; Guo, H.; Miller, J.T.; Kwak, J.H.; Peden, C.H.F.; Kiefer, B.; Allard, L.F.; et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 2014. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, M.; Takahashi, N.; Tanabe, T.; Nagai, Y.; Dohmae, K. Ideal Pt loading for a Pt/CeO2-based catalyst stabilized by a Pt–O–Ce bond. Appl. Catal. B Environ. 2010, 99, 336–342. [Google Scholar] [CrossRef]
- Chen, L.F.; Hu, J.C.; Richards, R. Intercalation of aggregation-free and well-dispersed gold nanoparticles into the walls of mesoporous silica as a robust “green” catalyst for n-alkane oxidation. J. Am. Chem. Soc. 2009, 131, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Jirkovsky, J.S.; Panas, I.; Ahlberg, E.; Halasa, M.; Romani, S.; Schiffrin, D.J. Single atom hot-spots at Au–Pd nanoalloys for electrocatalytic H2O2 Production. J. Am. Chem. Soc. 2011, 133, 19432–19441. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Allard, L.F.; Flytzani-Stephanopoulos, M. Atomically dispersed Au–(OH)x species bound on titania catalyze the low-temperature water-gas shift reaction. J. Am. Chem. Soc. 2013, 135, 3768–3771. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lee, S.Y. Production of succinic acid by bacterial fermentation. Enzyme Microb. Techol. 2006, 39, 352–361. [Google Scholar] [CrossRef]
- Binder, J.B.; Raines, R.T. Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Dai, W.L.; Fan, K. Remarkable support crystal phase effect in Au/FeOx catalyzed oxidation of 1, 4-butanediol to γ-butyrolactone. J. Catal. 2009, 266, 228–235. [Google Scholar] [CrossRef]
- Delhomme, C.; Weuster-Botz, D.; Kühn, F.E. Succinic acid from renewable resources as a C4 building-block chemical-a review of the catalytic possibilities in aqueous media. Green Chem. 2009, 11, 13–26. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Hong, U.G.; Hwang, S.; Seo, J.G.; Yi, J.; Song, I.K. Hydrogenation of succinic acid to γ-butyrolactone over palladium catalyst supported on mesoporous alumina xerogel. Catal. Lett. 2010, 138, 28–33. [Google Scholar] [CrossRef]
- Chung, S.H.; Park, Y.M.; Kim, M.S.; Lee, K.Y. The effect of textural properties on the hydrogenation of succinic acid using palladium incorporated mesoporous supports. Catal. Today 2012, 185, 205–210. [Google Scholar] [CrossRef]
- Tapin, B.; Epron, F.; Especel, C.; Ly, B.K.; Pinel, C.; Besson, M. Study of monometallic Pd/TiO2 catalysts for the hydrogenation of succinic acid in aqueous phase. ACS Catal. 2013, 3, 2327–2335. [Google Scholar] [CrossRef]
- Hong, U.G.; Park, H.W.; Lee, J.; Hwang, S.; Yi, J.; Song, I.K. Hydrogenation of succinic acid to tetrahydrofuran (THF) over rhenium catalyst supported on H2SO4-treated mesoporous carbon. Appl. Catal. A Gen. 2012, 415, 141–148. [Google Scholar] [CrossRef]
- Ly, B.K.; Minh, D.P.; Pinel, C.; Besson, M.; Tapin, B.; Epron, F.; Especel, C. Effect of addition mode of Re in bimetallic Pd-Re/TiO2 catalysts upon the selective aqueous-phase hydrogenation of succinic acid to 1, 4-Butanediol. Top Catal. 2012, 55, 466–473. [Google Scholar] [CrossRef]
- Hara, Y.; Kusaka, H.; Inagaki, H.; Takahashi, K.; Wada, K. A novel production of γ-butyrolactone catalyzed by ruthenium complexes. J. Catal. 2000, 194, 188–197. [Google Scholar] [CrossRef]
- Deshpande, R.M.; Buwa, V.V.; Rode, C.V.; Chaudhari, R.V.; Mills, P.L. Tailoring of activity and selectivity using bimetallic catalyst in hydrogenation of succinic acid. Catal. Commun. 2002, 3, 269–274. [Google Scholar] [CrossRef]
- Luque, R.; Lin, C.S.K.; Du, C.; Macquarrie, D.J.; Koutinas, A.; Wang, R.; Webb, C.; Clark, J.H. Chemical transformations of succinic acid recovered from fermentation broths by a novel direct vacuum distillation-crystallisation method. Green Chem. 2009, 11, 193–200. [Google Scholar] [CrossRef]
- Minh, P.; Besson, M.; Pinel, C.; Fuertes, P.; Petitjean, C. Aqueous-phase hydrogenation of biomass-based succinic acid to 1, 4-butanediol over supported bimetallic catalysts. Top. Catal. 2010, 53, 1270–1273. [Google Scholar] [CrossRef]
- Di, X.; Shao, Z.; Li, C.; Li, W.; Liang, C. Hydrogenation of succinic acid over supported rhenium catalysts prepared by the microwave-assisted thermolytic method. Catal. Sci. Technol. 2015, 5, 2441–2448. [Google Scholar] [CrossRef]
- Ly, B.K.; Tapin, B.; Aouine, M.; Delichere, P.; Epron, F.; Pinel, C.; Especel, C.; Besson, M. Insights into the oxidation state and location of rhenium in Re-Pd/TiO2 catalysts for aqueous-phase selective hydrogenation of succinic acid to 1,4-butanediol as a function of palladium and rhenium deposition methods. ChemCatChem 2015, 7, 2161–2178. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Hackett, S.F.J.; Brydson, R.M.; Gass, M.H.; Harvey, I.; Newman, A.D.; Wilson, K.; Lee, A.F. High-activity, single-site mesoporous Pd/Al2O3 catalysts for selective aerobic oxidation of allylic alcohols. Angew. Chem. Int. Ed. 2007, 46, 8593–8596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, L.F.; Cheng, H.Y.; Zhu, X.D.; Qi, Z.W. Atomically dispersed Pd catalysts for the selective hydrogenation of succinic acid to γ-butyrolactone. Catal. Today 2016. [Google Scholar] [CrossRef]
- Jentys, A. Estimation of mean size and shape of small metal particles by EXAFS. Phys. Chem. Chem. Phys. 1999, 1, 4059–4063. [Google Scholar] [CrossRef]
- Efremenko, I.; German, E.D.; Sheintuch, M. Density functional study of the interactions between dihydrogen and Pdn (n = 1–4) clusters. J. Phys. Chem. A 2000, 104, 8089–8096. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, J.; Wu, Z.; Zhang, Z. Microwave-assisted synthesis of various ZnO hierarchical nanostructures: Effects of heating parameters of microwave oven. Cryst. Growth Des. 2008, 8, 3148–3153. [Google Scholar] [CrossRef]
- Yang, G.; Kong, Y.; Hou, W.; Yan, Q. Heating behavior and crystal growth mechanism in microwave field. J. Phys. Chem. B 2005, 109, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Ugalde, M.; Chavira, E.; Figueroa, I.A.; Quintanar, C.; Espinosa-Magana, F.; Zaragoza-Contreras, E.A.; Ochoa-Lara, M.T. Preparation of rhodium nano-particles using microwaves. J. Sol Gel Sci. Technol. 2013, 65, 311–317. [Google Scholar] [CrossRef]
- You, C.J.; Zhang, C.; Chen, L.F.; Zhu, X.D.; Qi, Z.W. Highly dispersed palladium nanoclusters incorporated in amino-functionalized silica spheres for the selective hydrogenation of succinic acid to γ-butyrolactone. Appl. Organomet. Chem. 2015, 29, 653–660. [Google Scholar] [CrossRef]
- Hong, U.G.; Lee, J.; Hwang, S.; Song, I.K. Hydrogenation of succinic acid to γ-butyrolactone (GBL) over palladium-alumina composite catalyst prepared by a single-step sol-gel method. Catal. Lett. 2011, 141, 332–338. [Google Scholar] [CrossRef]
- Hong, U.G.; Hwang, S.; Seo, J.G.; Lee, J.; Song, I.K. Hydrogenation of succinic acid to γ-butyrolactone (GBL) over palladium catalyst supported on alumina xerogel: Effect of acid density of the catalyst. J. Ind. Eng. Chem. 2011, 17, 316–320. [Google Scholar] [CrossRef]
- Liu, Y.M.; Tsunoyama, H.; Akita, T.; Xie, S.; Tsukuda, T. Aerobic oxidation of cyclohexane catalyzed by size-controlled Au clusters on hydroxyapatite: Size effect in the sub-2 nm regime. ACS Catal. 2011, 1, 2–6. [Google Scholar] [CrossRef]
- Lei, Y.; Mehmood, F.; Lee, S.; Greeley, J.; Lee, B.; Seifert, S.; Winans, R.E.; Elam, J.W.; Meyer, R.J.; Redfern, P.C.; et al. Increased silver activity for direct propylene epoxidation via subnanometer size effects. Science 2010, 328, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, J.R.; Riva, A.; Peterson, E.J.; Bolin, T.; Datye, A.K. Improved low-temperature CO oxidation performance of Pd supported on La-stabilized alumina. ACS Catal. 2013, 3, 846–855. [Google Scholar] [CrossRef]
- Favera, N.D.; Kiehne, U.; Bunzen, J.; Hytteballe, S.; Lützen, A.; Piguet, C. Intermetallic interactions within solvated polynuclear complexes: A misunderstood concept. Angew. Chem. Int. Ed. 2010, 49, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Pulido, A.; Oliver-Tomas, B.; Renz, M.; Boronat, M.; Corma, A. Ketonic decarboxylation reaction mechanism: A combined experimental and DFT study. ChemSusChem 2013, 6, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chang, C.R.; Huang, Z.Q.; Li, J.; Wu, Z.; Ma, Y.; Qu, Y. High catalytic activity and chemoselectivity of sub-nanometric Pd clusters on porous-nanorods of CeO2 for hydrogenation of nitroarenes. J. Am. Chem. Soc. 2016, 138, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.M.; Rabelo-Neto, R.C.; Borges, L.E.; Jacobs, G.; Davis, B.H.; Graham, U.M.; Noronha, F.B. Effect of zirconia morphology on hydrodeoxygenation of phenol over Pd/ZrO2. ACS Catal. 2015, 5, 7385–7398. [Google Scholar] [CrossRef]
- Lopez-Acevedo, O.; Kacprzak, K.A.; Akola, J.; Häkkinen, H. Quantum size effects in ambient CO oxidation catalysed by ligand-protected gold clusters. Nat. Chem. 2010, 2, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, H.; Xu, B.Q. Catalysis by gold: Isolated surface Au3+ ions are active sites for selective hydrogenation of 1,3-butadiene over Au/ZrO2 catalysts. Angew. Chem. Int. Ed. 2005, 44, 7132–7135. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Hensen, E.J.M. Cyanide leaching of Au/CeO2: Highly active gold clusters for 1,3-butadiene hydrogenation. Phys. Chem. Chem. Phys. 2009, 11, 9578–9582. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, S.; Desimoni, J.; Rivas, P.C.; Cervera, M.M.; Caracoche, M.C. Hyperfine study on sol-gel derived-hematite doped zirconia. Chem. Mater. 2005, 17, 3486–3491. [Google Scholar] [CrossRef]
- Chen, L.F.; Hu, J.C.; Richards, R.M. Catalytic properties of nanoscale iron-doped zirconia solid-solution aerogels. ChemPhysChem 2008, 9, 1069–1078. [Google Scholar] [CrossRef] [PubMed]






| entries | Catalysts | Succinic acid Conversion (mol. %) a | γ-butyrolactone Selectivity (mol. %) b | Pd Dispersion (%) c | Pd Average size (nm) c | Turnover Frequencies (h−1) d |
|---|---|---|---|---|---|---|
| 1 | ZrO2 | 0 | 0 | - | - | - |
| 2 | 0.1 Pd/ZrO2 | 34 | 99 | 65.3 | 0.8 | 714 |
| 3 | 0.2 Pd/ZrO2 | 63 | 95 | 51.6 | 1.0 | 732 |
| 4 | 0.2 Pd/ZrO2 e | 67 | 94 | 51.6 | 1.0 | 780 |
| 5 | 0.2 Pd/ZrO2 f | 54 | 95 | 51.6 | 1.0 | 847 |
| 6 | 0.5 Pd/ZrO2 | 85 | 86 | 48.8 | 2.1 | 468 |
| 7 | 1.0 Pd/ZrO2 | 99 g | 85 g | 45.5 | 2.5 | 320 |
| 8 | 2.0%Pd/TiO2 h | 90 | 88 | 13.0 | - | 21 i |
| 9 | 2.1%Pd/TiO2 j | 94 | 90 | 28.0 | - | 6 i |
| 10 | 2.0%Ru/C k | 72 | 66 | - | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Cao, W.; Cheng, H.; Chen, L.; Qi, Z. Regenerable Subnanometer Pd Clusters on Zirconia for Highly Selective Hydrogenation of Biomass-Derived Succinic Acid in Water. Catalysts 2016, 6, 100. https://doi.org/10.3390/catal6070100
Zhang C, Cao W, Cheng H, Chen L, Qi Z. Regenerable Subnanometer Pd Clusters on Zirconia for Highly Selective Hydrogenation of Biomass-Derived Succinic Acid in Water. Catalysts. 2016; 6(7):100. https://doi.org/10.3390/catal6070100
Chicago/Turabian StyleZhang, Chi, Wenrong Cao, Hongye Cheng, Lifang Chen, and Zhiwen Qi. 2016. "Regenerable Subnanometer Pd Clusters on Zirconia for Highly Selective Hydrogenation of Biomass-Derived Succinic Acid in Water" Catalysts 6, no. 7: 100. https://doi.org/10.3390/catal6070100