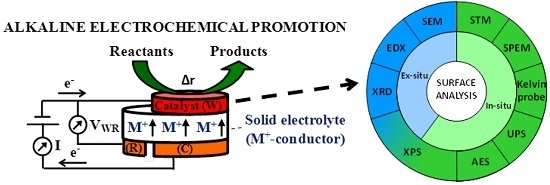
A Review of Surface Analysis Techniques for the Investigation of the Phenomenon of Electrochemical Promotion of Catalysis with Alkaline Ionic Conductors
Abstract
:1. General Features of Alkaline Electrochemical Promotion


2. Ex Situ Characterization of Alkali-Promoted Catalyst Surfaces


3. In Situ Characterization of Alkali-Promoted Catalyst Surfaces



4. Conclusions and Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mross, W.D. Alkali doping in heterogeneous catalysis. Catal. Rev. 1983, 25, 591–637. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Bartholomew, C.H. Fundamentals of Industrial Catalytic Processes; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Vayenas, C.G.; Bebelis, S.; Pliangos, C.S.; Brosda, D. Tsiplakides, Electrochemical Activation of Catalysis: Promotion, Electrochemical Promotion and Metal-Support Interactions; Kluwer Academic Publishers/Plenum Press: New York, NY, USA, 2001. [Google Scholar]
- Gellings, P.J.; Bouwmeester, H.J.M. The CRC Handbook of Solid State Electrochemistry; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Stoukides, M.; Vayenas, C.G. The effect of electrochemical oxygen pumping on the rate and selectivity of ethylene oxidation on polycrystalline silver. J. Catal. 1981, 70, 137–146. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A. New trends of Alkali Promotion in Heterogeneous Catalysis: Electrochemical Promotion with Alkaline Ionic Conductors. Catal. Surv. Asia 2015, 19, 25–37. [Google Scholar] [CrossRef]
- Vernoux, P.; Lizarraga, L.; Tsampas, M.N.; Sapountzi, F.M.; de Lucas-Consuegra, A.; Valverde, J.L.; Souentie, S.; Vayenas, C.G.; Tsiplakides, D.; Balomenou, S.; Baranova, E.A. Ionically conducting ceramics as active catalyst supports. Chem. Rev. 2013, 113, 8192–8260. [Google Scholar]
- Vayenas, C.G.; Bebelis, S.; Despotopoulou, M. Non-faradaic electrochemical modification of catalytic activity 4. The use of β″-Al2O3 as the solid electrolyte. J. Catal. 1991, 128, 415–435. [Google Scholar] [CrossRef]
- Karavasilis, C.; Bebelis, S.; Vayenas, C.G. In situ controlled promotion of catalyst surfaces via NEMCA: The effect of Na on the Ag-catalyzed ethylene epoxidation in the presence of chlorine moderators. J. Catal. 1996, 160, 205–213. [Google Scholar]
- Petrolekas, P.D.; Brosda, S.; Vayenas, C.G. Electrochemical promotion of Pt catalyst electrodes deposited on Na3Zr2Si2PO12 during ethylene oxidation. J. Electrochem. Soc. 1998, 145, 1469–1477. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Moggridge, G.; Vayenas, C.G.; Lambert, R.M. In Situ controlled promotion of catalyst surfaces via NEMCA: The effect of Na on the Pt-catalyzed CO oxidation. J. Catal. 1994, 146, 292–305. [Google Scholar] [CrossRef]
- Kotsionopoulos, N.; Bebelis, S. In situ electrochemical modification of catalytic activity for propane combustion of Pt/β-Al2O3 catalyst-electrodes. Top. Catal. 2007, 44, 379–389. [Google Scholar] [CrossRef]
- Vernoux, P.; Gaillard, F.; Lopez, C.; Siebert, E. In-situ electrochemical control of the catalytic activity of platinum for the propene oxidation. Solid State Ionics 2004, 175, 609–613. [Google Scholar] [CrossRef]
- Dorado, F.; de Lucas-Consuegra, A.; Vernoux, P.; Valverde, J.L. Electrochemical promotion of platinum impregnated catalyst for the selective catalytic reduction of NO by propene in presence of oxygen. Appl. Catal. B 2007, 73, 42–50. [Google Scholar] [CrossRef]
- Palermo, A.; Lambert, R.M.; Harkness, I.R.; Yentekakis, I.V.; Mar'ina, O.; Vayenas, C.G. Electrochemical promotion by Na of the platinum-catalyzed reaction between CO and NO. J. Catal. 1996, 161, 471–479. [Google Scholar] [CrossRef]
- Vernoux, P.; Gaillard, F.; Lopez, C.; Siebert, E. Coupling catalysis to electrochemistry: A solution to selective reduction of nitrogen oxides in lean-burn engine exhausts? J. Catal. 2003, 217, 203–208. [Google Scholar] [CrossRef]
- Williams, F.J.; Lambert, R.M. A study of sodium promotion in Fischer-Tropsch synthesis: Electrochemical control of a ruthenium model catalyst. Catal. Lett. 2000, 70, 9–14. [Google Scholar] [CrossRef]
- Cavalca, C.A.; Haller, G.L. Solid electrolytes as active catalyst supports: Electrochemical modification of benzene hydrogenation activity on Pt/β″(Na)Al2O3. J. Catal. 1998, 177, 389–395. [Google Scholar] [CrossRef]
- Bebelis, S.; Karasali, H.; Vayenas, C.G. Electrochemical promotion of the CO2 hydrogenation on Pd/YSZ and Pd/β″-Al2O3 catalyst-electrodes. Solid State Ionics 2008, 179, 1391–1395. [Google Scholar] [CrossRef]
- Pitselis, G.E.; Petrolekas, P.D.; Vayenas, C.G. Electrochemical promotion of ammonia decomposition over Fe catalyst films interfaced with K+- & H+- conductors. Ionics 1997, 3, 110–116. [Google Scholar]
- Urquhart, A.J.; Keel, J.M.; Williams, F.J.; Lambert, R.M. Electrochemical Promotion by Potassium of Rhodium-Catalyzed Fischer-Tropsch Synthesis: XP Spectroscopy and Reaction Studies. J. Phys. Chem. B 2003, 107, 10591–10597. [Google Scholar] [CrossRef]
- Urquhart, A.J.; Williams, F.J.; Lambert, R.M. Electrochemical promotion by potassium of Rh-catalysed fischer-tropsch synthesis at high pressure. Catal. Lett. 2005, 103, 137–141. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A.; Dorado, F.; Valverde, J.L.; Karoum, R.; Vernoux, P. Electrochemical activation of Pt catalyst by potassium for low temperature CO deep oxidation. Catal. Commun. 2008, 9, 17–20. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A.; Dorado, F.; Valverde, J.L.; Karoum, R.; Vernoux, P. Low-temperature propene combustion over Pt/K-βAl2O3 electrochemical catalyst: Characterization, catalytic activity measurements, and investigation of the NEMCA effect. J. Catal. 2007, 251, 474–484. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A.; Caravaca, A.; Dorado, F.; Valverde, J.L. Pt/K-βAl2O3 solid electrolyte cell as a “smart electrochemical catalyst” for the effective removal of NOx under wet reaction conditions. Catal. Today 2009, 146, 330–335. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A.; Dorado, F.; Jiménez-Borja, C.; Valverde, J.L. Influence of the reaction conditions on the electrochemical promotion by potassium for the selective catalytic reduction of N2O by C3H6 on platinum. Appl. Catal. B 2008, 78, 222–231. [Google Scholar] [CrossRef]
- Ruiz, E.; Cillero, D.; Martínez, P.J.; Morales, Á.; Vicente, G.S.; De Diego, G.; Sánchez, J.M. Bench scale study of electrochemically promoted catalytic CO2 hydrogenation to renewable fuels. Catal. Today 2013, 210, 55–66. [Google Scholar] [CrossRef]
- Ruiz, E.; Cillero, D.; Martínez, P.J.; Morales, Á.; Vicente, G.S.; de Diego, G.; Sánchez, J.M. Electrochemical synthesis of fuels by CO2 hydrogenation on Cu in a potassium ion conducting membrane reactor at bench scale. Catal. Today 2014, 236, 108–120. [Google Scholar] [CrossRef]
- Makri, M.; Katsaounis, A.; Vayenas, C.G. Electrochemical promotion of CO2 hydrogenation on Ru catalyst-electrodes supported on a K-β″-Al2O3 solid electrolyte. Electrochim. Acta 2015, 179, 556–564. [Google Scholar]
- Gutiérrez-Guerra, N.; González-Cobos, J.; Serrano-Ruiz, J.C.; Valverde, J.L.; de Lucas-Consuegra, A. Electrochemical Activation of Ni Catalysts with Potassium Ionic Conductors for CO2 Hydrogenation. Top. Catal. 2015, 58, 1256–1269. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A.; González-Cobos, J.; García-Rodríguez, Y.; Mosquera, A.; Endrino, J.L.; Valverde, J.L. Enhancing the catalytic activity and selectivity of the partial oxidation of methanol by electrochemical promotion. J. Catal. 2012, 293, 149–157. [Google Scholar] [CrossRef]
- González-Cobos, J.; Rico, V.J.; González-Elipe, A.R.; Valverde, J.L.; de Lucas-Consuegra, A. Electrochemical activation of an oblique angle deposited Cu catalyst film for H2 production. Catal. Sci. Tec. 2015, 5, 2203–2214. [Google Scholar] [CrossRef]
- González-Cobos, J.; López-Pedrajas, D.; Ruiz-López, E.; Valverde, J.L.; de Lucas-Consuegra, A. Applications of the Electrochemical Promotion of Catalysis in Methanol Conversion Processes. Top. Catal. 2015, 58, 1290–1302. [Google Scholar] [CrossRef]
- Harkness, I.R.; Hardacre, C.; Lambert, R.M.; Yentekakis, I.V.; Vayenas, C.G. Ethylene oxidation over platinum: In situ electrochemically controlled promotion using Na-β″ alumina and studies with a Pt(111)/Na model catalyst. J. Catal. 1996, 160, 19–26. [Google Scholar] [CrossRef]
- Konsolakis, M.; Palermo, A.; Tikhov, M.; Lambert, R.M.; Yentekakis, I.V. Electrochemical vs. conventional promotion: A new tool to design effective, highly dispersed conventional catalysts. Ionics 1998, 4, 148–156. [Google Scholar] [CrossRef]
- Lambert, R.M.; Tikhov, M.; Palermo, A.; Yentekakis, I.V.; Vayenas, C.G. Electrochemical promotion of environmentally important catalytic reactions. Ionics 1995, 1, 366–376. [Google Scholar] [CrossRef]
- Filkin, N.C.; Tikhov, M.S.; Palermo, A.; Lambert, R.M. A Kinetic and Spectroscopic Study of the in Situ Electrochemical Promotion by Sodium of the Platinum-Catalyzed Combustion of Propene. J. Phys. Chem. A 1999, 103, 2680–2687. [Google Scholar] [CrossRef]
- Williams, F.J.; Palermo, A.; Tracey, S.; Tikhov, M.S.; Lambert, R.M. Electrochemical promotion by potassium of the selective hydrogenation of acetylene on platinum: Reaction studies and XP spectroscopy. J. Phys. Chem. B 2002, 106, 5668–5672. [Google Scholar] [CrossRef]
- Williams, F.J.; Palermo, A.; Tikhov, M.S.; Lambert, R.M. Electrochemical promotion by sodium of the rhodium-catalyzed reduction of NO by propene: Kinetics and spectroscopy. J. Phys. Chem. B 2001, 105, 1381–1388. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Palermo, A.; Filkin, N.C.; Tikhov, M.S.; Lambert, R.M. In situ electrochemical promotion by sodium of the platinum-catalyzed reduction of NO by propene. J. Phys. Chem. B 1997, 101, 3759–3768. [Google Scholar] [CrossRef]
- Williams, F.J.; Palermo, A.; Tikhov, M.S.; Lambert, R.M. Electrochemical promotion by sodium of the rhodium-catalyzed NO + CO reaction. J. Phys. Chem. B 2000, 104, 11883–11890. [Google Scholar] [CrossRef]
- Williams, F.J.; Palermo, A.; Tikhov, M.S.; Lambert, R.M. Mechanism of alkali promotion in heterogeneous catalysis under realistic conditions: Application of electron spectroscopy and electrochemical promotion to the reduction of NO by CO and by propene over rhodium. Surf. Sci. 2001, 482–485, 177–182. [Google Scholar]
- Williams, F.J.; Tikhov, M.S.; Palermo, A.; Macleod, N.; Lambert, R.M. Electrochemical promotion of rhodium-catalyzed NO reduction by CO and by propene in the presence of oxygen. J. Phys. Chem. B 2002, 105, 2800–2808. [Google Scholar] [CrossRef]
- Lambert, R.M.; Williams, F.; Palermo, A.; Tikhov, M.S. Modelling alkali promotion in heterogeneous catalysis: In situ electrochemical control of catalytic reactions. Top. Catal. 2000, 13, 91–98. [Google Scholar] [CrossRef]
- Williams, F.J.; Palermo, A.; Tikhov, M.S.; Lambert, R.M. First Demonstration of in Situ Electrochemical Control of a Base Metal Catalyst: Spectroscopic and Kinetic Study of the CO + NO Reaction over Na-Promoted Cu. J.Phys. Chem. B 1999, 103, 9960–9966. [Google Scholar] [CrossRef]
- Williams, F.J.; Palermo, A.; Tikhov, M.S.; Lambert, R.M. The Origin of Electrochemical Promotion in Heterogeneous Catalysis: Photoelectron Spectroscopy of Solid State Electrochemical Cells. J. Phys. Chem. B 2000, 104, 615–621. [Google Scholar] [CrossRef]
- Carley, A.F.; Roberts, M.W. X-ray photoelectron spectroscopic study of the interaction of oxygen and nitric oxide with aluminium. Proc. R. Soc. London Ser. A 1978, 363, 403–424. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Bebelis, S.; Ladas, S. Dependence of catalytic rates on catalyst work function. Nature 1990, 343, 625–627. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Lambert, R.M.; Tikhov, M.S.; Konsolakis, M.; Kiousis, V. Promotion by sodium in emission control catalysis: A kinetic and spectroscopic study of the Pd-catalyzed reduction of NO by propene. J. Catal. 1998, 176, 82–92. [Google Scholar] [CrossRef]
- Günther, S.; Kolmakov, A.; Kovac, J.; Kiskinova, M. Artefact formation in scanning photoelectron emission microscopy. Ultramicroscopy 1998, 75, 35–51. [Google Scholar] [CrossRef]
- Makri, M.; Vayenas, G.G.; Bebelis, S.; Besocke, K.H.; Cavalca, C. Atomic resolution STM imaging of electrochemically controlled reversible promoter dosing of catalysts. Surf. Sci. 1996, 369, 351–359. [Google Scholar] [CrossRef]
- Makri, M.; Vayenas, C.G.; Bebelis, S.; Besocke, K.H.; Cavalca, C. Atomic resolution scanning tunneling microscopy imaging of Pt electrodes interfaced with β″-Al2O3. Ionics 1996, 2, 248–253. [Google Scholar] [CrossRef]
- Archonta, D.; Frantzis, A.; Tsiplakides, D.; Vayenas, C.G. STM observation of the origin of electrochemical promotion on metal catalyst-electrodes interfaced with YSZ and β″-Al2O3. Solid State Ionics 2006, 177, 2221–2225. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cobos, J.; De Lucas-Consuegra, A. A Review of Surface Analysis Techniques for the Investigation of the Phenomenon of Electrochemical Promotion of Catalysis with Alkaline Ionic Conductors. Catalysts 2016, 6, 15. https://doi.org/10.3390/catal6010015
González-Cobos J, De Lucas-Consuegra A. A Review of Surface Analysis Techniques for the Investigation of the Phenomenon of Electrochemical Promotion of Catalysis with Alkaline Ionic Conductors. Catalysts. 2016; 6(1):15. https://doi.org/10.3390/catal6010015
Chicago/Turabian StyleGonzález-Cobos, Jesús, and Antonio De Lucas-Consuegra. 2016. "A Review of Surface Analysis Techniques for the Investigation of the Phenomenon of Electrochemical Promotion of Catalysis with Alkaline Ionic Conductors" Catalysts 6, no. 1: 15. https://doi.org/10.3390/catal6010015