Heterostructured S-TiO2/g-C3N4 Photocatalysts with High Visible Light Photocatalytic Activity
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization of S-TiO2/g-C3N4 Catalysts
2.2. Photocatalytic Activity
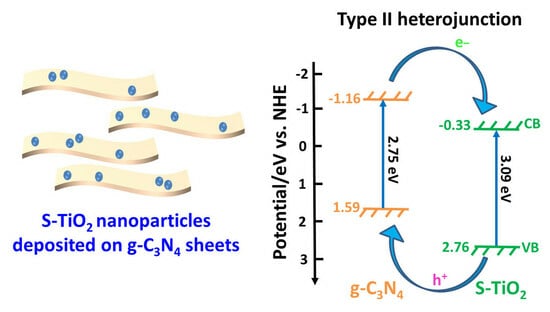
2.3. Reaction Mechanism
3. Materials and Methods
3.1. Reagents
3.2. Preparation of TiS2
3.3. Synthesis of g-C3N4
3.4. Preparation of S-TiO2/g-C3N4 Photocatalysts
3.5. Photocatalytic Tests
3.6. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashid, R.; Shafiq, I.; Akhter, P.; Javid Iqbal, M.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Arya, S.; Kumar, S. Industrial wastewater treatment: Current trends, bottlenecks, and best practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef] [PubMed]
- Adisurya Ismail, G.; Sakai, H. Review on effect of different type of dyes on advanced oxidation processes (AOPs) for textile color removal. Chemosphere 2022, 291, 132906. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A comprehensive review on reactive oxygen species (ROS) in advanced oxidation processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [CrossRef]
- Moussa, H.; Girot, E.; Mozet, K.; Alem, H.; Medjahdi, G.; Schneider, R. ZnO rods/reduced graphene oxide composites prepared via a solvothermal reaction for efficient sunlight-driven photocatalysis. Appl. Catal. B Environ. 2016, 185, 11–21. [Google Scholar] [CrossRef]
- Moussa, H.; Chouchene, B.; Gries, T.; Balan, L.; Mozet, K.; Medjahdi, G.; Schneider, R. Growth of ZnO Nanorods on Graphitic Carbon Nitride gCN Sheets for the Preparation of Photocatalysts with High Visible-Light Activity. ChemCatChem 2018, 10, 4987–4997. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: Preparation, modification and environmental application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; An, X.; Hou, L.-a. A critical review of g-C3N4-based photocatalytic membrane for water purification. Chem. Eng. J. 2021, 412, 128663. [Google Scholar] [CrossRef]
- Ouedraogo, S.; Chouchene, B.; Desmarets, C.; Gries, T.; Balan, L.; Fournet, R.; Medjahdi, G.; Bayo, K.; Schneider, R. Copper octacarboxyphthalocyanine as sensitizer of graphitic carbon nitride for efficient dye degradation under visible light irradiation. Appl. Catal. A Gen. 2018, 563, 127–136. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, B.; Fan, J.; Ho, W.; Yu, J. g-C3N4-Based 2D/2D Composite Heterojunction Photocatalyst. Small Struct. 2021, 2, 2100086. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent advances in g-C3N4-based heterojunction photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Chouchene, B.; Gries, T.; Balan, L.; Medjahdi, G.; Schneider, R. Graphitic carbon nitride/SmFeO3 composite Z-scheme photocatalyst with high visible light activity. Nanotechnology 2020, 31, 465704. [Google Scholar] [CrossRef]
- Acharya, R.; Parida, K. A review on TiO2/g-C3N4 visible-light-responsive photocatalysts for sustainable energy generation and environmental remediation. J. Environ. Chem. Eng. 2020, 8, 103896. [Google Scholar] [CrossRef]
- Tatykayev, B.; Chouchene, B.; Balan, L.; Gries, T.; Medjahdi, G.; Girot, E.; Uralbekov, B.; Schneider, R. Heterostructured g-CN/TiO2 Photocatalysts Prepared by Thermolysis of g-CN/MIL-125(Ti) Composites for Efficient Pollutant Degradation and Hydrogen Production. Nanomaterials 2020, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Song, L.; Luo, L.; Zhang, Y.; Zhu, B.; Liu, J.; Chen, Z.; Zhang, L. Preparation of TiO2/C3N4 heterojunctions on carbon-fiber cloth as efficient filter-membrane-shaped photocatalyst for removing various pollutants from the flowing wastewater. J. Colloid Interface Sci. 2018, 532, 798–807. [Google Scholar] [CrossRef]
- Kane, A.; Chafiq, L.; Dalhatou, S.; Bonnet, P.; Nasr, M.; Gaillard, N.; Dangwang Dikdim, J.M.; Monier, G.; Amine Assadi, A.; Zeghioud, H. g-C3N4/TiO2 S-scheme heterojunction photocatalyst with enhanced photocatalytic Carbamazepine degradation and mineralization. J. Photochem. Photobiol. A Chem. 2022, 430, 113971. [Google Scholar] [CrossRef]
- Zhang, B.; He, X.; Ma, X.; Chen, Q.; Liu, G.; Zhou, Y.; Ma, D.; Cui, C.; Ma, J.; Xin, Y. In situ synthesis of ultrafine TiO2 nanoparticles modified g-C3N4 heterojunction photocatalyst with enhanced photocatalytic activity. Sep. Purif. Technol. 2020, 247, 116932. [Google Scholar] [CrossRef]
- Hu, K.; Li, R.; Ye, C.; Wang, A.; Wei, W.; Hu, D.; Qiu, R.; Yan, K. Facile synthesis of Z-scheme composite of TiO2 nanorod/g-C3N4 nanosheet efficient for photocatalytic degradation of ciprofloxacin. J. Clean. Prod. 2020, 253, 120055. [Google Scholar] [CrossRef]
- Hao, R.; Wang, G.; Tang, H.; Sun, L.; Xu, C.; Han, D. Template-free preparation of macro/mesoporous g-C3N4/TiO2 heterojunction photocatalysts with enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2016, 187, 47–58. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Qu, J.; Chen, Y.; Tad, M.O.; Shao, Z. Synthesis of Hierarchical TiO2–C3N4 Hybrid Microspheres with Enhanced Photocatalytic and Photovoltaic Activities by Maximizing the Synergistic Effect. ChemPhotoChem 2017, 1, 35–45. [Google Scholar] [CrossRef]
- Cao, Y.; Yuan, G.; Guo, Y.; Hu, X.; Fang, G.; Wang, S. Facile synthesis of TiO2/g-C3N4 nanosheet heterojunctions for efficient photocatalytic degradation of tartrazine under simulated sunlight. Appl. Surf. Sci. 2022, 600, 154169. [Google Scholar] [CrossRef]
- Ni, S.; Fu, Z.; Li, L.; Ma, M.; Liu, Y. Step-scheme heterojunction g-C3N4/TiO2 for efficient photocatalytic degradation of tetracycline hydrochloride under UV light. Colloids Surf. A Physicochem. Eng. 2022, 649, 129475. [Google Scholar] [CrossRef]
- Liu, X.; Chen, N.; Li, Y.; Deng, D.; Xing, X.; Wang, Y. A general nonaqueous sol-gel route to g-C3N4-coupling photocatalysts: The case of Z-scheme g-C3N4/TiO2 with enhanced photodegradation toward RhB under visible-light. Sci. Rep. 2016, 6, 39531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Mei, J.; Sarina, S.; Wu, Z.; Liao, T.; Yan, C.; Suna, Z. Strongly interfacial-coupled 2D-2D TiO2/g-C3N4 heterostructure for enhanced visible-light induced synthesis and conversion. J. Hazard. Mater. 2020, 394, 122529. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, Z.; Chen, Q.; Cao, C.; Zhao, Y.; Yang, W.; Zeng, L.; Huang, L. Fabrication of 0D/2D TiO2 Nanodots/g-C3N4 S-scheme heterojunction photocatalyst for efficient photocatalytic overall water splitting. Appl. Surf. Sci. 2022, 571, 151287. [Google Scholar] [CrossRef]
- Yang, J.; Wu, X.; Mei, Z.; Zhou, S.; Su, Y.; Wang, G. CVD Assisted Synthesis of Macro/Mesoporous TiO2/g-C3N4 S-Scheme Heterojunction for Enhanced Photocatalytic Hydrogen Evolution. Adv. Sustain. Syst. 2002, 6, 220005. [Google Scholar] [CrossRef]
- Zhang, X.; Ping Jiang, S. Layered g-C3N4/TiO2 nanocomposites for efficient photocatalytic water splitting and CO2 reduction: A review. Mater. Today Energy 2022, 23, 100904. [Google Scholar] [CrossRef]
- Reli, M.; Huo, P.; Sihor, M.; Ambrozova, N.; Troppova, I.; Matejova, L.; Lang, J.; Svoboda, L.; Kustrowski, P.; Ritz, M.; et al. Novel TiO2/C3N4 Photocatalysts for Photocatalytic Reduction of CO2 and for Photocatalytic Decomposition of N2O. J. Phys. Chem. A 2016, 120, 8564–8573. [Google Scholar] [CrossRef]
- Mukit Hossain, S.; Park, H.; Kang, H.-J.; Seok Mun, J.; Tijing, L.; Rhee, I.; Kim, J.-H.; Jun, Y.-S.; Kyong Shon, H. Modified Hydrothermal Route for Synthesis of Photoactive Anatase TiO2/g-CN Nanotubes from Sludge Generated TiO2. Catalysts 2020, 10, 1350. [Google Scholar] [CrossRef]
- Chen, X.; Burda, C. The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. J. Am. Chem. Soc. 2008, 130, 5018–5019. [Google Scholar] [CrossRef]
- Tachikawa, T.; Tojo, S.; Kawai, K.; Endo, M.; Fujitsuka, M.; Ohno, T.; Nishijima, K.; Miyamoto, Z.; Majima, T. Photocatalytic oxidation reactivity of holes in the sulfur and carbon-doped TiO2 powders studied by time-resolved diffuse reflectance spectroscopy. J. Phys. Chem. B 2004, 108, 19299–19306. [Google Scholar] [CrossRef]
- Ohno, T.; Mitsui, T.; Matsumura, M. Photocatalytic Activity of S-doped TiO2 Photocatalyst under Visible Light. Chem. Lett. 2003, 32, 364–365. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Band gap narrowing of titanium dioxide by sulfur doping. Appl. Phys. Lett. 2002, 81, 454. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Tanaka, S.; Asai, K. Visible Light-Induced Degradation of Methylene Blue on S-doped TiO2. Chem. Lett. 2003, 32, 330–331. [Google Scholar] [CrossRef]
- Ho, W.; Yu, J.C.; Lee, S. Low-temperature hydrothermal synthesis of S-doped TiO2 with visible light photocatalytic activity. J. Solid State Chem. 2006, 179, 1171–1176. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chien, T.-E.; Lai, P.-C.; Chiang, Y.-H.; Li, K.-L.; Lin, J.-L. TiS2 transformation into S-doped and N-doped TiO2 with visible-light catalytic activity. Appl. Surf. Sci. 2015, 359, 1–6. [Google Scholar] [CrossRef]
- Pany, S.K.; Parida, M. A facile in situ approach to fabricate N,S-TiO2/g-C3N4 nanocomposite with excellent activity for visible light induced water splitting for hydrogen evolution. Phys. Chem. Chem. Phys. 2015, 17, 8070–8077. [Google Scholar] [CrossRef] [PubMed]
- Biswal, L.; Nayak, S.; Parida, K. Rationally designed Ti3C2/N,S-TiO2/g-C3N4 ternary heterostructure with spatial charge separation for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2022, 621, 254–266. [Google Scholar] [CrossRef]
- Mukit Hossain, S.; Park, H.; Kang, H.-J.; Seok Mun, J.; Tijing, L.; Rhee, I.; Kim, J.-H.; Jun, Y.-S.; Kyong Shon, H. Synthesis and NOx removal performance of anatase S-TiO2/g-CN heterojunction formed from dye wastewater sludge. Chemosphere 2021, 275, 130020. [Google Scholar] [CrossRef]
- Lopez, J.; Gonzalez, R.; Ayala, J.; Cantu, J.; Castillo, A.; Parsons, J.; Myers, J.; Lodge, T.P.; Alcoutlabi, M. Centrifugally spun TiO2/C composite fibers prepared from TiS2/PAN precursor fibers as binder-free anodes for LIBS. J. Phys. Chem. Solids 2021, 149, 109795. [Google Scholar] [CrossRef]
- Yang, G.; Yanb, Z.; Xiao, T. Low-temperature solvothermal synthesis of visible-light-responsive S-doped TiO2 nanocrystal. Appl. Surf. Sci. 2012, 258, 4016–4022. [Google Scholar] [CrossRef]
- Yang, J.; Bai, H.; Tan, X.; Lian, J. IR and XPS investigation of visible-light photocatalysis-Nitrogen-carbon-doped TiO2 film. Appl. Surf. Sci. 2006, 253, 1988–1994. [Google Scholar] [CrossRef]
- She, X.; Liu, L.; Ji, H.; Mo, Z.; Li, Y.; Huang, L.; Du, D.; Xu, H.; Li, H. Template-free synthesis of 2D porous ultrathin nonmetal-doped g-C3N4 nanosheets with highly efficient photocatalytic H2 evolution from water under visible light. Appl. Catal. B Environ. 2016, 187, 144–153. [Google Scholar] [CrossRef]
- Chen, X.; Glans, P.-A.; Qiu, X.; Dayal, S.; Jennings, W.D.; Smith, K.E.; Burda, C.; Guo, J. X-Ray Spectroscopic Study of the Electronic Structure of Visible-light Responsive N-, C- and S-Doped TiO2. J. Electron Spectrosc. Relat. Phenom. 2008, 162, 67–73. [Google Scholar] [CrossRef]
- McManamon, C.; O’Connell, J.; Delaney, P.; Rasappa, S.; Holmes, J.D.; Morris, M.A. A facile route to synthesis of S-doped TiO2 nanoparticles for photocatalytic activity. J. Mol. Catal. A Chem. 2015, 406, 51–57. [Google Scholar] [CrossRef]
- Zhang, T.; Oyama, T.; Aoshima, A.; Hidaka, H.; Zhao, J.; Serpone, N. Photooxidative N-demethylation of methylene blue in aqueous TiO2 dispersions under UV irradiation. J. Photochem. Photobiol. Chem. 2001, 140, 163–172. [Google Scholar] [CrossRef]
- Matos, J.; Laine, J.; Herrmann, J.-M. Synergy effect in the photocatalytic degradation of phenol on a suspended mixture of titania and activated carbon. Appl. Catal. B Environ. 1998, 18, 281–291. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef]
- Fernández-Nieves, A.; De las Nieves, F.J.; Richter, C. Trends in Colloid and Interface Science XII; Steinkopff: Dresden, Germany, 1998; pp. 21–24. [Google Scholar]
- Xiuling, G.; Jihai, D.; Chaojie, L.; Zisheng, Z.; Weiwen, W. Fabrication of g-C3N4/TiO2 photocatalysts with a special bilayer structure for visible light photocatalytic application. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124931. [Google Scholar]
- Pelin, G.; Jongee, P.; Abdullah, Ö. Preparation and photocatalytic activity of g-C3N4/TiO2 heterojunctions under solar light illumination. Ceram. Int. 2020, 46, 21431–21438. [Google Scholar]
- Yarabahally, R.G.; Udayabhanu; Gubran, A.; Abdo, H.; Mysore, B.N.; Ganganagappa, N.; Kullaiah, B. Facile and rapid synthesis of solar-driven TiO2/g-C3N4 heterostructure photocatalysts for enhanced photocatalytic activity. J. Sci. Adv. Mater. Devices 2022, 7, 100419. [Google Scholar]
- Sepide, S.; Bahram, K.; Zohreh, B. Synthesis and characterization of a g-C3N4/TiO2-ZnO nanostructure for photocatalytic degradation of methylene blue. Nano Futures 2022, 6, 035001. [Google Scholar]
- Tianyi, C.; Yuan, Z.; Yumin, Y.; Jianbo, Z.; Kezhen, Q.; Jianhui, J. Synthesis and properties of Sm-TiO2 coupled with g-C3N4 for improved photocatalytic degradation toward methylene blue and tetracycline under visible-light irradiation. Appl. Organomet. Chem. 2022, 36, e6626. [Google Scholar]
- Dang, T.N.H.; Nguyen, T.T.T.; Huynh, Q.A.T.; Le Van, T.S.; Le Vu, T.S.; Nguyen, D.V.Q.; Le Lam, S.; Tran, N.T.; Pham, L.M.T.; Ly, H.D.; et al. TiO2/g-C3N4 Visible-Light-Driven Photocatalyst for Methylene Blue Decomposition. J. Nanomater. 2023, 2023, 9967890. [Google Scholar]
- Sadia, F.; Umair, A.; Muhammad, B.T.; Wahid, A.; Muhammad, A.; Muhammad, S. Spinach derived boron-doped g-C3N4/TiO2 composites for efficient photo-degradation of methylene blue dye. Chemosphere 2023, 320, 138002. [Google Scholar]
- Vijayan, M.; Manikandan, V.; Rajkumar, C.; Hatamleh, A.A.; Alnafisi, B.K.; Easwaran, G.; Liu, X.; Sivakumar, K.; Kim, H. Constructing Z-scheme g-C3N4/TiO2 heterostructure for promoting degradation of the hazardous dye pollutants. Chemosphere 2023, 311, 136928. [Google Scholar] [CrossRef]
- Xiaoting, R.; Mingshuai, G.; Lili, X.; Likun, X.; Li, L.; Lehui, Y.; Min, W.; Yonglei, X.; Fangyuan, D.; Yadi, W. Photoelectrochemical performance and S-scheme mechanism of ternary GO/g-C3N4/TiO2 heterojunction photocatalyst for photocatalytic antibiosis and dye degradation under visible light. Appl. Surf. Sci. 2023, 630, 157446. [Google Scholar]
- Shuhua, L.; Xinxin, S.; Ying, W.; Boyang, J.; Caixia, S.; Debao, W. Construction of coordination bond electron bridge boosting interfacial electron transfer to enhance bifunctional photocatalytic activity of TiO2/g-C3N4 heterojunction. Mater. Today Commun. 2023, 37, 107605. [Google Scholar]
- Jiayi, W.; Penggang, R.; Yanli, D.; Xueyan, Z.; Zhengyan, C.; Lu, P.; Yanling, J. Construction of tubular g-C3N4/TiO2 S-scheme photocatalyst for high-efficiency degradation of organic pollutants under visible light. J. Alloys Compd. 2023, 947, 169659. [Google Scholar]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]











| Photocatalyst | Photocatalyst Amount (g/L) | [MB] (mg/L) | Light Source | Removal (%) | Reaction Time (min) | Ref |
|---|---|---|---|---|---|---|
| g-C3N4/TiO2 | Immobilized catalyst | 10 | Visible light | 97.6 | 240 | [51] |
| g-C3N4/TiO2 | 0.1 | 10 | Solar light illumination | 80 | 180 | [52] |
| TiO2/g-C3N4 | Not provided | 5 | Simulated sunlight | 84.6 | 120 | [53] |
| g-C3N4/TiO2-ZnO | 1 | 10 | Visible light | 62.4 | 120 | [54] |
| Sm-doped TiO2/g-C3N4 | 1 | 1000 | Visible light | 91.8 | 120 | [55] |
| TiO2/g-C3N4 | 0.2 | 10 | Visible light | 100 | 70 | [56] |
| B-doped g-C3N4/TiO2 | 1 | Not provided | Visible light | 81 | 225 | [57] |
| g-C3N4/TiO2 | 0.2 | 30 | Simulated sunlight | 100 | 120 | [58] |
| GO/g-C3N4/TiO2 | 0.1 | 10 | Visible light | 98.8 | 240 | [59] |
| TiO2/g-C3N4 | 0.3 | 10 | Simulated sunlight | 100 | 60 | [60] |
| g-C3N4/TiO2 | 0.4 | 20 | Visible light | 96.6 | 60 | [61] |
| S-doped TiO2/g-C3N4 | 1 | 10 | Visible light | 100 | 240 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaya, Y.; Chouchene, B.; Medjahdi, G.; Balan, L.; Bouguila, N.; Schneider, R. Heterostructured S-TiO2/g-C3N4 Photocatalysts with High Visible Light Photocatalytic Activity. Catalysts 2024, 14, 226. https://doi.org/10.3390/catal14040226
Alaya Y, Chouchene B, Medjahdi G, Balan L, Bouguila N, Schneider R. Heterostructured S-TiO2/g-C3N4 Photocatalysts with High Visible Light Photocatalytic Activity. Catalysts. 2024; 14(4):226. https://doi.org/10.3390/catal14040226
Chicago/Turabian StyleAlaya, Yassine, Bilel Chouchene, Ghouti Medjahdi, Lavinia Balan, Noureddine Bouguila, and Raphaël Schneider. 2024. "Heterostructured S-TiO2/g-C3N4 Photocatalysts with High Visible Light Photocatalytic Activity" Catalysts 14, no. 4: 226. https://doi.org/10.3390/catal14040226