Groundwater Bioremediation through Reductive Dechlorination in a Permeable Bioelectrochemical Reactor
Abstract
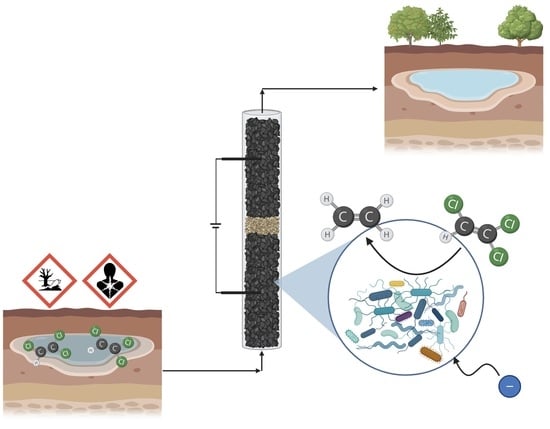
:1. Introduction
2. Results and Discussion
2.1. Groundwater Sampling and Characterisation
2.2. Fluid Dynamic Characterisation and Preliminary Abiotic Tests
2.3. Synthetic Groundwater Laboratory Test
3. Material and Methods
3.1. Bioelectrochemical Membrane-Less PBR-like Reactor
3.2. Bioelectrochemical Reactor Operating Conditions
3.3. Analytical Procedures
3.4. Data Elaboration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradley, P.M. History and Ecology of Chloroethene Biodegradation: A Review. Bioremediation J. 2003, 7, 81–109. [Google Scholar] [CrossRef]
- Moran, M.J.; Zogorski, J.S.; Squillace, P.J. Chlorinated Solvents in Groundwater of the United States. Environ. Sci. Technol. 2007, 41, 74–81. [Google Scholar] [CrossRef] [PubMed]
- McCarty, P.L. Groundwater Contamination by Chlorinated Solvents: History, Remediation Technologies and Strategies. In In Situ Remediation of Chlorinated Solvent Plumes; Stroo, H.F., Ward, C.H., Eds.; Springer: New York, NY, USA, 2010; pp. 1–28. [Google Scholar] [CrossRef]
- Jugder, B.-E.; Ertan, H.; Bohl, S.; Lee, M.; Marquis, C.P.; Manefield, M. Organohalide Respiring Bacteria and Reductive Dehalogenases: Key Tools in Organohalide Bioremediation. Front. Microbiol. 2016, 7, 181210. [Google Scholar] [CrossRef] [PubMed]
- Atashgahi, S.; Häggblom, M.M.; Smidt, H. Organohalide respiration in pristine environments: Implications for the natural halogen cycle. Environ. Microbiol. 2018, 20, 934–948. [Google Scholar] [CrossRef] [PubMed]
- Atashgahi, S.; Lu, Y.; Smidt, H. Overview of Known Organohalide-Respiring Bacteria—Phylogenetic Diversity and Environmental Distribution. In Organohalide-Respiring Bacteria; Adrian, L., Löffler, F.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 63–105. [Google Scholar] [CrossRef]
- He, J.; Ritalahti, K.M.; Yang, K.-L.; Koenigsberg, S.S.; Löffler, F.E. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 2003, 424, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Löffler, F.E.; Yan, J.; Ritalahti, K.M.; Adrian, L.; Edwards, E.A.; Konstantinidis, K.T.; Müller, J.A.; Fullerton, H.; Zinder, S.H.; Spormann, A.M. Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 2, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Lohner, S.T.; Deutzmann, J.S.; Logan, B.E.; Leigh, J.; Spormann, A.M. Hydrogenase-independent uptake and metabolism of electrons by the archaeon Methanococcus maripaludis. ISME J. 2014, 8, 1673–1681. [Google Scholar] [CrossRef]
- Roubaud, E.; Lacroix, R.; Da Silva, S.; Bergel, A.; Basséguy, R.; Erable, B. Catalysis of the hydrogen evolution reaction by hydrogen carbonate to decrease the voltage of microbial electrolysis cell fed with domestic wastewater. Electrochim. Acta 2018, 275, 32–39. [Google Scholar] [CrossRef]
- Lacroix, R.; Roubaud, E.; Erable, B.; Etcheverry, L.; Bergel, A.; Basséguy, R.; Da Silva, S. Design of 3D microbial anodes for microbial electrolysis cells (MEC) fuelled by domestic wastewater. Part I: Multiphysics modelling. J. Environ. Chem. Eng. 2021, 9, 105476. [Google Scholar] [CrossRef]
- Cristiani, L.; Zeppilli, M.; Porcu, C.; Majone, M. Ammonium Recovery and Biogas Upgrading in a Tubular Micro-Pilot Microbial Electrolysis Cell (MEC). Molecules 2020, 25, 2723. [Google Scholar] [CrossRef]
- Ceballos-Escalera, A.; Pous, N.; Balaguer, M.D.; Puig, S. Electrochemical water softening as pretreatment for nitrate electro bioremediation. Sci. Total Environ. 2022, 806, 150433. [Google Scholar] [CrossRef]
- Wang, X.; Aulenta, F.; Puig, S.; Esteve-Núñez, A.; He, Y.; Mu, Y.; Rabaey, K. Microbial electrochemistry for bioremediation. Environ. Sci. Ecotechnol. 2020, 1, 100013. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Aulenta, F.; Villano, M.; Angenent, L.T. Cathodes as electron donors for microbial metabolism: Which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 2011, 102, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Majone, M.; Verdini, R.; Aulenta, F.; Rossetti, S.; Tandoi, V.; Kalogerakis, N.; Agathos, S.; Puig, S.; Zanaroli, G.; Fava, F. In situ groundwater and sediment bioremediation: Barriers and perspectives at European contaminated sites. New Biotechnol. 2015, 32, 133–146. [Google Scholar] [CrossRef]
- Verdini, R.; Aulenta, F.; de Tora, F.; Lai, A.; Majone, M. Relative contribution of set cathode potential and external mass transport on TCE dechlorination in a continuous-flow bioelectrochemical reactor. Chemosphere 2015, 136, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Jiang, W.; Chen, D.; Xu, Y. Bioremediation of typical chlorinated hydrocarbons by microbial reductive dechlorination and its key players: A review. Ecotoxicol. Environ. Saf. 2020, 202, 110925. [Google Scholar] [CrossRef] [PubMed]
- Aulenta, F.; Maio, V.D.; Ferri, T.; Majone, M. The humic acid analogue antraquinone-2,6-disulfonate (AQDS) serves as an electron shuttle in the electricity-driven microbial dechlorination of trichloroethene to cis-dichloroethene. Bioresour. Technol. 2010, 101, 9728–9733. [Google Scholar] [CrossRef]
- Rabaey, K.; Angenent, L.; Schröder, U.; Keller, J. Bioelectrochemical systems: From extracellular electron transfer to biotechnological application. Water Intell. Online 2009, 8, 9781780401621. [Google Scholar] [CrossRef]
- Tiehm, A.; Schmidt, K.R. Sequential anaerobic/aerobic biodegradation of chloroethenes—Aspects of field application. Curr. Opin. Biotechnol. 2011, 22, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Mattes, T.E.; Alexander, A.K.; Coleman, N.V. Aerobic biodegradation of the chloroethenes: Pathways, enzymes, ecology, and evolution. FEMS Microbiol. Rev. 2010, 34, 445–475. [Google Scholar] [CrossRef]
- Mattes, T.E.; Jin, Y.O.; Livermore, J.; Pearl, M.; Liu, X. Abundance and activity of vinyl chloride (VC)-oxidizing bacteria in a dilute groundwater VC plume biostimulated with oxygen and ethene. Appl. Microbiol. Biotechnol. 2015, 99, 9267–9276. [Google Scholar] [CrossRef] [PubMed]
- Lohner, S.T.; Becker, D.; Mangold, K.-M.; Tiehm, A. Sequential Reductive and Oxidative Biodegradation of Chloroethenes Stimulated in a Coupled Bioelectro-Process. Environ. Sci. Technol. 2011, 45, 6491–6497. [Google Scholar] [CrossRef]
- Pant, P.; Pant, S. A review: Advances in microbial remediation of trichloroethylene (TCE). J. Environ. Sci. 2010, 22, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, L.; Gu, J.-D. Microbial electrocatalysis: Redox mediators responsible for extracellular electron transfer. Biotechnol. Adv. 2018, 36, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Extracellular electron transfer: Wires, capacitors, iron lungs, and more. Geobiology 2008, 6, 225–231. [Google Scholar] [CrossRef]
- Vogel, M.; Kopinke, F.-D.; Mackenzie, K. Acceleration of microiron-based dechlorination in water by contact with fibrous activated carbon. Sci. Total Environ. 2019, 660, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Hoareau, M.; Etcheverry, L.; Chapleur, O.; Bureau, C.; Midoux, C.; Erable, B.; Bergel, A. The electrochemical microbial tree: A new concept for wastewater treatment. Chem. Eng. J. 2023, 454, 140295. [Google Scholar] [CrossRef]
- Gonzalez-Nava, C.; Manríquez, J.; Godínez, L.A.; Rodríguez-Valadez, F.J. Enhancement of the electron transfer and ion transport phenomena in microbial fuel cells containing humic acid-modified bioanodes. Bioelectrochemistry 2022, 144, 108003. [Google Scholar] [CrossRef]
- Aulenta, F.; Catervi, A.; Majone, M.; Panero, S.; Reale, P.; Rossetti, S. Electron Transfer from a Solid-State Electrode Assisted by Methyl Viologen Sustains Efficient Microbial Reductive Dechlorination of TCE. Environ. Sci. Technol. 2007, 41, 2554–2559. [Google Scholar] [CrossRef]
- Lai, A.; Verdini, R.; Aulenta, F.; Majone, M. Influence of nitrate and sulfate reduction in the bioelectrochemically assisted dechlorination of cis-DCE. Chemosphere 2015, 125, 147–154. [Google Scholar] [CrossRef]
- Zeppilli, M.; Dell’Armi, E.; Cristiani, L.; Papini, M.P.; Majone, M. Reductive/Oxidative Sequential Bioelectrochemical Process for Perchloroethylene Removal. Water 2019, 11, 2579. [Google Scholar] [CrossRef]
- Tucci, M.; Fernández-Verdejo, D.; Resitano, M.; Ciacia, P.; Guisasola, A.; Blánquez, P.; Marco-Urrea, E.; Viggi, C.C.; Matturro, B.; Crognale, S.; et al. Toluene-driven anaerobic biodegradation of chloroform in a continuous-flow bioelectrochemical reactor. Chemosphere 2023, 338, 139467. [Google Scholar] [CrossRef] [PubMed]
- Dell’Armi, E.; Zeppilli, M.; De Santis, F.; Papini, M.P.; Majone, M. Control of Sulfate and Nitrate Reduction by Setting Hydraulic Retention Time and Applied Potential on a Membraneless Microbial Electrolysis Cell for Perchloroethylene Removal. ACS Omega 2021, 6, 25211–25218. [Google Scholar] [CrossRef] [PubMed]
- Dell’Armi, E.; Zeppilli, M.; Di Franca, M.L.; Matturro, B.; Feigl, V.; Molnár, M.; Berkl, Z.; Németh, I.; Yaqoubi, H.; Rossetti, S.; et al. Evaluation of a bioelectrochemical reductive/oxidative sequential process for chlorinated aliphatic hydrocarbons (CAHs) removal from a real contaminated groundwater. J. Water Process Eng. 2022, 49, 103101. [Google Scholar] [CrossRef]
- Field, J.A.; Sierra-Alvarez, R. Biodegradability of chlorinated solvents and related chlorinated aliphatic compounds. Rev. Environ. Sci. Biotechnol. 2004, 3, 185–254. [Google Scholar] [CrossRef]
- Lin, H.-W.; Kustermans, C.; Vaiopoulou, E.; Prévoteau, A.; Rabaey, K.; Yuan, Z.; Pikaar, I. Electrochemical oxidation of iron and alkalinity generation for efficient sulfide control in sewers. Water Res. 2017, 118, 114–120. [Google Scholar] [CrossRef]
- Zeikus, J.G. The biology of methanogenic bacteria. Bacteriol. Rev. 1977, 41, 514–541. [Google Scholar] [CrossRef]
- Balch, W.E.; Fox, G.E.; Magrum, L.J.; Woese, C.R.; Wolfe, R.S. Methanogens: Reevaluation of a unique biological group. Microbiol. Rev. 1979, 43, 260–296. [Google Scholar] [CrossRef]








| μmol/L | μg/L | (CSC) μg/L | |
|---|---|---|---|
| VC | 0.34 ± 0.10 | 21.1 | 0.5 |
| cisDCE | 0.98 ± 0.12 | 61.1 | 60 |
| TCE | 0.59 ± 0.3 | 77.3 | 1.5 |
| PCE | 0.09 ± 0.01 | 15.2 | 1.1 |
| Chloride | 800 ± 16 | 2910 ± 31 | |
| Nitrate | 400 ± 21 | 2630 ± 40 | |
| Sulphate | 2013 ± 40 | 1895 ± 210 | 2500 |
| PRB | Internal Counter-Electrode [35] | |
|---|---|---|
| HRT (d−1) | 0.8 | 1.2 |
| RD (meq/Ld) | 20 ± 11 | 134 ± 11 |
| CE RD (%) | 0.1 ± 0.1 | 2.1 ± 0.5 |
| CE NO3−(%) | 17 ± 2 | 12 ± 4 |
| CE SO4−2(%) | 22 ± 4 | 89 ± 7 |
| Energy consumption (kWh/m3) | 4.6 ± 0.9 | 0.6 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sassetto, G.; Lorini, L.; Lai, A.; Petrangeli Papini, M.; Zeppilli, M. Groundwater Bioremediation through Reductive Dechlorination in a Permeable Bioelectrochemical Reactor. Catalysts 2024, 14, 208. https://doi.org/10.3390/catal14030208
Sassetto G, Lorini L, Lai A, Petrangeli Papini M, Zeppilli M. Groundwater Bioremediation through Reductive Dechlorination in a Permeable Bioelectrochemical Reactor. Catalysts. 2024; 14(3):208. https://doi.org/10.3390/catal14030208
Chicago/Turabian StyleSassetto, Geremia, Laura Lorini, Agnese Lai, Marco Petrangeli Papini, and Marco Zeppilli. 2024. "Groundwater Bioremediation through Reductive Dechlorination in a Permeable Bioelectrochemical Reactor" Catalysts 14, no. 3: 208. https://doi.org/10.3390/catal14030208