A Self-Disperse Copper-Based Catalyst Synthesized via a Dry Mixing Method for Acetylene Hydrochlorination
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Change of CuX + C during Acetylene Hydrochlorination
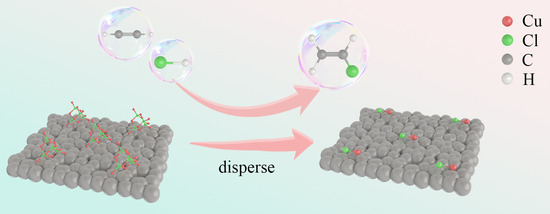
2.2. The Reaction-Induced Dispersion of CuCl on Activated Carbon
2.3. The Effects of Temperature on the Performance of CuCl + C Catalysts
2.4. Effect of the Amount and Dispersion of CuCl on CuCl + C Catalyst Performance
2.5. Comparison between Dry Mixing Method and Impregnation Method
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalyst Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. Heterogeneous Catalysis: Understanding for Designing, and Designing for Applications. Angew. Chem. Int. Ed. 2016, 55, 6112–6113. [Google Scholar] [CrossRef]
- Sun, X.; Dawson, S.R.; Parmentier, T.E.; Malta, G.; Davies, T.E.; He, Q.; Lu, L.; Morgan, D.J.; Carthey, N.; Johnston, P.; et al. Facile Synthesis of Precious-Metal Single-Site Catalysts Using Organic Solvents. Nat. Chem. 2020, 12, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, S.K.; Chen, Z.; Faust Akl, D.; Mitchell, S.; Pérez-Ramírez, J. Single-Atom Catalysts across the Periodic Table. Chem. Rev. 2020, 120, 11703–11809. [Google Scholar] [CrossRef]
- Malta, G.; Freakley, S.J.; Kondrat, S.A.; Hutchings, G.J. Acetylene Hydrochlorination Using Au/Carbon: A Journey towards Single Site Catalysis. Chem. Commun. 2017, 53, 11733–11746. [Google Scholar] [CrossRef]
- Johnston, P.; Carthey, N.; Hutchings, G.J. Discovery, Development, and Commercialization of Gold Catalysts for Acetylene Hydrochlorination. J. Am. Chem. Soc. 2015, 137, 14548–14557. [Google Scholar] [CrossRef] [PubMed]
- Malta, G.; Kondrat, S.A.; Freakley, S.J.; Davies, C.J.; Lu, L.; Dawson, S.; Thetford, A.; Gibson, E.K.; Morgan, D.J.; Jones, W.; et al. Identification of Single-Site Gold Catalysis in Acetylene Hydrochlorination. Science 2017, 355, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Amrute, A.P.; Pérez-Ramírez, J. Halogen-Mediated Conversion of Hydrocarbons to Commodities. Chem. Rev. 2017, 117, 4182–4247. [Google Scholar] [CrossRef]
- Ciacci, L.; Passarini, F.; Vassura, I. The European PVC Cycle: In-Use Stock and Flows. Resour. Conserv. Recycl. 2017, 123, 108–116. [Google Scholar] [CrossRef]
- Trotuş, I.-T.; Zimmermann, T.; Schüth, F. Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited. Chem. Rev. 2014, 114, 1761–1782. [Google Scholar] [CrossRef]
- Faust Akl, D.; Giannakakis, G.; Ruiz-Ferrando, A.; Agrachev, M.; Medrano-García, J.D.; Guillén-Gosálbez, G.; Jeschke, G.; Clark, A.H.; Safonova, O.V.; Mitchell, S.; et al. Reaction-Induced Formation of Stable Mononuclear Cu(I)Cl Species on Carbon for Low-Footprint Vinyl Chloride Production. Adv. Mater. 2023, 35, 2211464. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Q.; Chen, K.; Wang, Y.; Huang, C.; Dai, H.; Yu, F.; Kang, L.; Dai, B. Development of a Heterogeneous Non-Mercury Catalyst for Acetylene Hydrochlorination. ACS Catal. 2015, 5, 5306–5316. [Google Scholar] [CrossRef]
- Kaiser, S.K.; Lin, R.; Mitchell, S.; Fako, E.; Krumeich, F.; Hauert, R.; Safonova, O.V.; Kondratenko, V.A.; Kondratenko, E.V.; Collins, S.M.; et al. Controlling the Speciation and Reactivity of Carbon-Supported Gold Nanostructures for Catalysed Acetylene Hydrochlorination. Chem. Sci. 2019, 10, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, G. Vapor Phase Hydrochlorination of Acetylene: Correlation of Catalytic Activity of Supported Metal Chloride Catalysts. J. Catal. 1985, 96, 292–295. [Google Scholar] [CrossRef]
- Ye, L.; Duan, X.; Wu, S.; Wu, T.-S.; Zhao, Y.; Robertson, A.W.; Chou, H.-L.; Zheng, J.; Ayvalı, T.; Day, S.; et al. Self- Regeneration of Au/CeO2 Based Catalysts with Enhanced Activity and Ultra-Stability for Acetylene Hydrochlorination. Nat. Commun. 2019, 10, 914. [Google Scholar] [CrossRef]
- Kaiser, S.K.; Fako, E.; Surin, I.; Krumeich, F.; Kondratenko, V.A.; Kondratenko, E.V.; Clark, A.H.; López, N.; Pérez-Ramírez, J. Performance Descriptors of Nanostructured Metal Catalysts for Acetylene Hydrochlorination. Nat. Nanotechnol. 2022, 17, 606–612. [Google Scholar] [CrossRef]
- Kaiser, S.K.; Fako, E.; Manzocchi, G.; Krumeich, F.; Hauert, R.; Clark, A.H.; Safonova, O.V.; López, N.; Pérez-Ramírez, J. Nanostructuring Unlocks High Performance of Platinum Single-Atom Catalysts for Stable Vinyl Chloride Production. Nat. Catal. 2020, 3, 376–385. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, J.; Yu, L.; Jin, Y.; Li, W. Active Ruthenium Species in Acetylene Hydrochlorination. Appl. Catal. A Gen. 2014, 488, 28–36. [Google Scholar] [CrossRef]
- Zhang, J.; Sheng, W.; Guo, C.; Li, W. Acetylene Hydrochlorination over Bimetallic Ru-Based Catalysts. RSC Adv. 2013, 3, 21062–21068. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, J.; Zhang, T.; Di, X.; Gu, S.; Ni, J.; Li, X. Ultra-Low Ru-Promoted CuCl2 as Highly Active Catalyst for the Hydrochlorination of Acetylene. RSC Adv. 2015, 5, 38159–38163. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, H.; Liu, Z.; Sun, S.; Huang, W.; Qu, Z.; Yan, N. Tunable Redox Cycle and Enhanced π-Complexation in Acetylene Hydrochlorination over RuCu Catalysts. ACS Catal. 2022, 12, 7579–7588. [Google Scholar] [CrossRef]
- Zhou, K.; Si, J.; Jia, J.; Huang, J.; Zhou, J.; Luo, G.; Wei, F. Reactivity Enhancement of N-CNTs in Green Catalysis of C2H2 Hydrochlorination by a Cu Catalyst. RSC Adv. 2014, 4, 7766–7769. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, T.; Liu, Y.; Li, W.; Zhang, H.; Zhang, J. Phosphine-Oxide Organic Ligand Improved Cu-Based Catalyst for Acetylene Hydrochlorination. Appl. Catal. A Gen. 2022, 630, 118461. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Zhang, P.; Wang, Q.; Zhu, M.; Dai, B. Nitrogen and Phosphorus Co-Doped Activated Carbon Induces High Density Cu+ Active Center for Acetylene Hydrochlorination. Chin. J. Chem. Eng. 2023, 59, 193–199. [Google Scholar] [CrossRef]
- Wang, Y.; Nian, Y.; Zhang, J.; Li, W.; Han, Y. MOMTPPC Improved Cu-Based Heterogeneous Catalyst with High Efficiency for Acetylene Hydrochlorination. Mol. Catal. 2019, 479, 110612. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, M.; Dai, B. The Preparation of Cu-g-C3N4/AC Catalyst for Acetylene Hydrochlorination. Catalysts 2016, 6, 193. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhao, J.; Di, X.; Di, S.; Wang, B.; Yue, Y.; Sheng, G.; Lai, H.; Guo, L.; Wang, H.; et al. Carbon-Supported Perovskite-like CsCuCl3 Nanoparticles: A Highly Active and Cost-Effective Heterogeneous Catalyst for the Hydrochlorination of Acetylene to Vinyl Chloride. Catal. Sci. Technol. 2018, 8, 2901–2908. [Google Scholar] [CrossRef]
- Wang, B.; Jin, C.; Shao, S.; Yue, Y.; Zhang, Y.; Wang, S.; Chang, R.; Zhang, H.; Zhao, J.; Li, X. Electron-Deficient Cu Site Catalyzed Acetylene Hydrochlorination. Green Energy Environ. 2023, 8, 1128–1140. [Google Scholar] [CrossRef]
- Xie, Y.-C.; Tang, Y.-Q. Spontaneous Monolayer Dispersion of Oxides and Salts onto Surfaces of Supports: Applications to Heterogeneous Catalysis. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 1990; Volume 37, pp. 1–43. ISBN 978-0-12-007837-0. [Google Scholar]
- Xiao, F.-S.; Zheng, S.; Sun, J.; Yu, R.; Qiu, S.; Xu, R. Dispersion of Inorganic Salts into Zeolites and Their Pore Modification. J. Catal. 1998, 176, 474–487. [Google Scholar] [CrossRef]
- Wang, C.-B.; Cai, Y.; Wachs, I.E. Reaction-Induced Spreading of Metal Oxides onto Surfaces of Oxide Supports during Alcohol Oxidation: Phenomenon, Nature, and Mechanisms. Langmuir 1999, 15, 1223–1235. [Google Scholar] [CrossRef]
- Morgan, K.; Goguet, A.; Hardacre, C. Metal Redispersion Strategies for Recycling of Supported Metal Catalysts: A Perspective. ACS Catal. 2015, 5, 3430–3445. [Google Scholar] [CrossRef]
- Bennett, E.; Wilson, T.; Murphy, P.J.; Refson, K.; Hannon, A.C.; Imberti, S.; Callear, S.K.; Chass, G.A.; Parker, S.F. How the Surface Structure Determines the Properties of CuH. Inorg. Chem. 2015, 54, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Malta, G.; Kondrat, S.A.; Freakley, S.J.; Morgan, D.J.; Gibson, E.K.; Wells, P.P.; Aramini, M.; Gianolio, D.; Thompson, P.B.J.; Johnston, P.; et al. In Situ K-Edge X-ray Absorption Spectroscopy of the Ligand Environment of Single-Site Au/C Catalysts during Acetylene Hydrochlorination. Chem. Sci. 2020, 11, 7040–7052. [Google Scholar] [CrossRef] [PubMed]






| Catalyst | Cu (%) | Recovery (%) |
|---|---|---|
| 5% CuCl + C | 5.41 | - |
| 5% CuCl + C-u-20 h-180 °C 1 | 5.25 | 97.0 |
| 5% CuCl + C-u-110 h-180 °C | 5.15 | 95.2 |
| 5% CuCl + C-u-20 h-190 °C | 5.11 | 94.6 |
| 5% CuCl + C-u-20 h-200 °C | 4.98 | 92.2 |
| 5% CuCl + C-u-20 h-210 °C | 4.75 | 87.8 |
| 5% CuCl/C | 5.50 | - |
| 5% CuCl/C-u-20 h-200 °C | 5.15 | 93.6 |
| 5% CuCl + CD 2 | 5.25 | - |
| Catalyst | STotal (m2/g) | SMicro (m2/g) | VMicro (cm3/g) |
|---|---|---|---|
| 5% CuCl + C | 1047.11 | 1022.85 | 0.405 |
| 5% CuCl + CD | 995 | 936.8 | 0.366 |
| 5% CuCl/C | 904.36 | 880.95 | 0.349 |
| 5% CuCl + C-u-20 h-180 °C * | 905.5 | 875.8 | 0.345 |
| 5% CuCl + C-u-48 h-180 °C | 724.7 | 664.8 | 0.261 |
| 5% CuCl + C-u-110 h-180 °C | 611.6 | 569.8 | 0.226 |
| 5% CuCl + C-u-20 h-190 °C | 883.4 | 834.6 | 0.326 |
| 5% CuCl + C-u-20 h-200 °C | 855.9 | 799.9 | 0.313 |
| 5% CuCl + C-u-20 h-210 °C | 701.66 | 672.15 | 0.270 |
| 1% CuCl + C-u-20 h-200 °C | 1036 | 999.5 | 0.393 |
| 3% CuCl + C-u-20 h-200° C | 961.3 | 917.9 | 0.361 |
| 7% CuCl + C-u-20 h-200 °C | 716.04 | 689.6 | 0.272 |
| 10% CuCl + C-u-20 h-200 °C | 497.3 | 471.7 | 0.189 |
| 5% CuCl + CD-u-20 h-200 °C | 741.6 | 696.7 | 0.276 |
| 5% CuCl/C-u-20 h-200 °C | 557.6 | 533.9 | 0.216 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Sun, X.; Zhang, J.; Huang, J. A Self-Disperse Copper-Based Catalyst Synthesized via a Dry Mixing Method for Acetylene Hydrochlorination. Catalysts 2024, 14, 207. https://doi.org/10.3390/catal14030207
Fu Y, Sun X, Zhang J, Huang J. A Self-Disperse Copper-Based Catalyst Synthesized via a Dry Mixing Method for Acetylene Hydrochlorination. Catalysts. 2024; 14(3):207. https://doi.org/10.3390/catal14030207
Chicago/Turabian StyleFu, Yuru, Xi Sun, Jian Zhang, and Jiahui Huang. 2024. "A Self-Disperse Copper-Based Catalyst Synthesized via a Dry Mixing Method for Acetylene Hydrochlorination" Catalysts 14, no. 3: 207. https://doi.org/10.3390/catal14030207