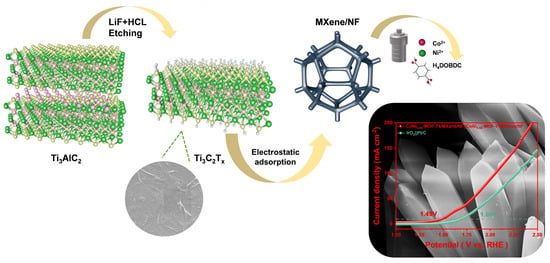
Ni Doped Co-MOF-74 Synergized with 2D Ti3C2Tx MXene as an Efficient Electrocatalyst for Overall Water-Splitting
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Synthesis and Characterization
2.2. Oxygen Evolution Reaction Performance
2.3. Hydrogen Evolution Reaction Performance
2.4. Overall Water Splitting Performance
3. Experimental Section
3.1. Materials and Chemicals
3.2. Sample Synthesis
3.2.1. Synthesis and Delamination of Ti3C2Tx MXene
3.2.2. Synthesis of MXene/NF
3.2.3. Synthesis of CoNi0.04-MOF-74/MXene/NF
3.2.4. Synthesis of CoNi0.04-MOF-74/NF
3.2.5. Synthesis of Pt/C @NF or IrO2@NF
3.3. Materials Characterization
3.4. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Hou, C.; Chen, C.; Ma, W.; Li, Q.; Hu, L.; Lv, X.; Dang, J. Collaborative Interface Optimization Strategy Guided Ultrafine RuCo and MXene Heterostructure Electrocatalysts for Efficient Overall Water Splitting. ACS Nano 2023, 17, 10947–10957. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shen, J.; Li, Q.; Zheng, X.; Wang, Z.; Cui, L.; Xu, J.; Liu, J. In-situ growth of VS4 nanorods on Ni-Fe sulfides nanoplate array towards achieving a highly efficient and bifunctional electrocatalyst for total water splitting. Chem. Eng. J. 2023, 474, 145461. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, J.; Guo, M. La-doped NiFe-LDH coupled with hierarchical vertically aligned MXene frameworks for efficient overall water splitting. J. Energy Chem. 2022, 70, 472–479. [Google Scholar] [CrossRef]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, 2201099. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yu, Z.; Zhang, Y.; Niu, S.; Zhao, J.; Li, S.; Xu, P. Understanding the Effect of Second Metal on CoM (M = Ni, Cu, Zn) Metal–Organic Frameworks for Electrocatalytic Oxygen Evolution Reaction. Small 2021, 17, 2105150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-J.; Ji, W.; Xu, L.; Yang, Y.; Wang, W.; Ding, H.; Xu, X.; Wang, W.; Zhang, P.; Hua, Z.; et al. Controllable transformation of CoNi-MOF-74 on Ni foam into hierarchical-porous Co(OH)2/Ni(OH)2 micro-rods with ultra-high specific surface area for energy storage. Chem. Eng. J. 2022, 428, 132123. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, B.; Li, S.; Zhou, L.; Lai, L.; Wang, Z.; Zhao, S.; Han, M.; Gao, K.; Lu, M.; et al. Interdiffusion Reaction-Assisted Hybridization of Two-Dimensional Metal–Organic Frameworks and Ti3C2Tx Nanosheets for Electrocatalytic Oxygen Evolution. ACS Nano 2017, 11, 5800–5807. [Google Scholar] [CrossRef]
- Lu, X.F.; Fang, Y.; Luan, D.; Lou, X.W.D. Metal–Organic Frameworks Derived Functional Materials for Electrochemical Energy Storage and Conversion: A Mini Review. Nano Lett. 2021, 21, 1555–1565. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating metal–organic frameworks for catalyzing acidic oxygen evolution for proton exchange membrane water electrolysis. SusMat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Romero-Muñiz, C.; Gavira-Vallejo, J.M.; Merkling, P.J.; Calero, S. Impact of Small Adsorbates in the Vibrational Spectra of Mg- and Zn-MOF-74 Revealed by First-Principles Calculations. ACS Appl. Mater. Interfaces 2020, 12, 54980–54990. [Google Scholar] [CrossRef]
- Vornholt, S.M.; Duncan, M.J.; Warrender, S.J.; Semino, R.; Ramsahye, N.A.; Maurin, G.; Smith, M.W.; Tan, J.-C.; Miller, D.N.; Morris, R.E. Multifaceted Study of the Interactions between CPO-27-Ni and Polyurethane and Their Impact on Nitric Oxide Release Performance. ACS Appl. Mater. Interfaces 2020, 12, 58263–58276. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Derakhshandeh, P.G.; Depauw, H.; Coudert, F.-X.; Vrielinck, H.; Van Der Voort, P.; Leus, K. Mixed-metal metal–organic frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Tarriño, S.; Olloqui-Sariego, J.L.; Calvente, J.J.; Palomino, M.; Espallargas, G.M.; Jordá, J.L.; Rey, F.; Oña-Burgos, P. Cobalt Metal–Organic Framework Based on Two Dinuclear Secondary Building Units for Electrocatalytic Oxygen Evolution. ACS Appl. Mater. Interfaces 2019, 11, 46658–46665. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Gao, J.; Zhang, Q. Recent advances in vacancy engineering of metal-organic frameworks and their derivatives for electrocatalysis. SusMat 2021, 1, 66–87. [Google Scholar] [CrossRef]
- Yan, L.; Cao, L.; Dai, P.; Gu, X.; Liu, D.; Li, L.; Wang, Y.; Zhao, X. Metal-oganic Frameworks Derived Nanotube of Nickel–Cobalt Bimetal Phosphides as Highly Efficient Electrocatalysts for Overall Water Splitting. Adv. Funct. Mater. 2017, 27, 1703455. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Metal–organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Liu, C.; Bai, Y.; Li, W.; Yang, F.; Zhang, G.; Pang, H. In Situ Growth of Three-Dimensional MXene/Metal-Organic Framework Composites for High-Performance Supercapacitors. Angew. Chem. Int. Ed. Engl. 2022, 61, e202116282. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, H.; Kong, F.; Tian, H.; Meng, G.; Wang, S.; Mao, X.; Cui, X.; Hou, X.; Shi, J. V2C MXene synergistically coupling FeNi LDH nanosheets for boosting oxygen evolution reaction. Appl. Catal. B Environ. 2021, 297, 120474. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Chen, W.; Yuan, R.; Zhan, K.; Zhu, M.; Yang, J.; Zhao, B. Fe3O4 nanoplates anchored on Ti3C2Tx MXene with enhanced pseudocapacitive and electrocatalytic properties. Nanoscale 2021, 13, 15343–15351. [Google Scholar] [CrossRef]
- Yue, X.; Dong, Y.; Cao, H.; Wei, X.; Zheng, Q.; Sun, W.; Lin, D. Effect of electronic structure modulation and layer spacing change of NiAl layered double hydroxide nanoflowers caused by cobalt doping on supercapacitor performance. J. Colloid Interface Sci. 2023, 630, 973–983. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Li, D.; Wang, Y.; Duan, C. Magnesium-regulated oxygen vacancies of nickel layered double hydroxides for electrocatalytic water oxidation. J. Mater. Chem. A 2018, 6, 18378–18383. [Google Scholar] [CrossRef]
- Feng, X.; Jiao, Q.; Chen, W.; Dang, Y.; Dai, Z.; Suib, S.L.; Zhang, J.; Zhao, Y.; Li, H.; Feng, C. Cactus-like NiCo2S4@NiFe LDH hollow spheres as an effective oxygen bifunctional electrocatalyst in alkaline solution. Appl. Catal. B Environ. 2021, 286, 119869. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Li, H.; Xia, Z.; Yu, S.; Wang, S.; Sun, G. Iron-based binary metal-organic framework nanorods as an efficient catalyst for the oxygen evolution reaction. Chin. J. Catal. 2021, 42, 637–647. [Google Scholar] [CrossRef]
- Zhou, J.; Han, Z.; Wang, X.; Gai, H.; Chen, Z.; Guo, T.; Hou, X.; Xu, L.; Hu, X.; Huang, M.; et al. Discovery of Quantitative Electronic Structure-OER Activity Relationship in Metal-Organic Framework Electrocatalysts Using an Integrated Theoretical-Experimental Approach. Adv. Funct. Mater. 2021, 31, 2102066. [Google Scholar] [CrossRef]
- Wang, S.; Li, Q.; Sun, S.; Ge, K.; Zhao, Y.; Yang, K.; Zhang, Z.; Cao, J.; Lu, J.; Yang, Y.; et al. Heterostructured ferroelectric BaTiO3@MOF-Fe/Co electrocatalysts for efficient oxygen evolution reaction. J. Mater. Chem. A 2022, 10, 5350–5360. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Kang, H.-S.; N’Diaye, A.T.; Lee, M.H. Lattice oxygen-mediated NiOOM formation for efficient oxygen evolution reaction in MOF@LDH core–shell structures. Chem. Eng. J. 2023, 454, 140403. [Google Scholar] [CrossRef]
- Papadakis, G.; Tsortos, A.; Kordas, A.; Tiniakou, I.; Morou, E.; Vontas, J.; Kardassis, D.; Gizeli, E. Acoustic detection of DNA conformation in genetic assays combined with PCR. Sci. Rep. 2013, 3, 2033. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, L.; Yang, Z.; Chang, Y.-M.; Hu, F.; Li, L.; Li, L.; Chen, H.-Y.; Peng, S. Electronic Modulation of Metal–Organic Frameworks by Interfacial Bridging for Efficient pH-Universal Hydrogen Evolution. Adv. Funct. Mater. 2023, 33, 2210322. [Google Scholar] [CrossRef]
- Karlsson, L.H.; Birch, J.; Halim, J.; Barsoum, M.W.; Persson, P.O.Å. Atomically Resolved Structural and Chemical Investigation of Single MXene Sheets. Nano Lett. 2015, 15, 4955–4960. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, J.; Jiang, Y.; Wu, Y.; Liu, B.; Chu, X.; Liu, C.; Che, G.; Lu, Y. Multivalent ruthenium immobilized by self-supported NiFe–organic frameworks for efficient electrocatalytic overall water splitting. J. Mater. Chem. A 2023, 11, 2769–2779. [Google Scholar] [CrossRef]
- Strauss, I.; Mundstock, A.; Hinrichs, D.; Himstedt, R.; Knebel, A.; Reinhardt, C.; Dorfs, D.; Caro, J. The Interaction of Guest Molecules with Co-MOF-74: A Vis/NIR and Raman Approach. Angew. Chem. Int. Ed. 2018, 57, 7434–7439. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shi, Y.; Wang, Z.; Liu, C.; Bi, J.; Yu, Y.; Wu, L. Unsaturated NiII Centers Mediated the Coordination Activation of Benzylamine for Enhancing Photocatalytic Activity over Ultrathin Ni MOF-74 Nanosheets. ACS Appl. Mater. Interfaces 2021, 13, 61286–61295. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Han, C.; Gao, J.; Pan, L.; Wu, J.; Zhu, X.-D.; Zou, J.-J. NiCo-Based Electrocatalysts for the Alkaline Oxygen Evolution Reaction: A Review. ACS Catal. 2021, 11, 12485–12509. [Google Scholar] [CrossRef]
- Shi, J.; Qiu, F.; Yuan, W.; Guo, M.; Lu, Z.-H. Nitrogen-doped carbon-decorated yolk-shell CoP@FeCoP micro-polyhedra derived from MOF for efficient overall water splitting. Chem. Eng. J. 2021, 403, 126312. [Google Scholar] [CrossRef]
- Tan, P.; Gao, R.; Zhang, Y.; Han, N.; Jiang, Y.; Xu, M.; Bao, S.-J.; Zhang, X. Electrostatically directed assembly of two-dimensional ultrathin Co2Ni-MOF/Ti3C2Tx nanosheets for electrocatalytic oxygen evolution. J. Colloid Interface Sci. 2023, 630, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Qi, R.; Yu, K.; Zhu, Z. Ultrathin Ti2NTx MXene-wrapped MOF-derived CoP frameworks towards hydrogen evolution and water oxidation. Electrochim. Acta 2021, 393, 139068. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Lv, Z.; Ma, W.; Wang, M.; Li, Q.; Dang, J. Constructing heterostructures of ZIF-67 derived C, N doped Co2P and Ti2VC2Tx MXene for enhanced OER. J. Mater. Sci. Technol. 2023, 145, 74–82. [Google Scholar] [CrossRef]
- Yue, Q.; Sun, J.; Chen, S.; Zhou, Y.; Li, H.; Chen, Y.; Zhang, R.; Wei, G.; Kang, Y. Hierarchical Mesoporous MXene–NiCoP Electrocatalyst for Water-Splitting. ACS Appl. Mater. Interfaces 2020, 12, 18570–18577. [Google Scholar] [CrossRef]
- Du, C.-F.; Dinh, K.N.; Liang, Q.; Zheng, Y.; Luo, Y.; Zhang, J.; Yan, Q. Self-Assemble and In Situ Formation of Ni1−xFexPS3 Nanomosaic-Decorated MXene Hybrids for Overall Water Splitting. Adv. Energy Mater. 2018, 8, 1801127. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, R.; Huang, X.; Xu, W.; Zhou, S.; Wei, Y.; Han, S.; Li, Y. In-situ derived Mo-doped NiCoP and MXene to form Mott-Schottky heterojunction with tunable surface electron density to promote overall water splitting. Compos. Part B Eng. 2023, 263, 110834. [Google Scholar] [CrossRef]
- Liu, D.; Xu, H.; Wang, C.; Shang, H.; Yu, R.; Wang, Y.; Li, J.; Li, X.; Du, Y. 3D Porous Ru-Doped NiCo-MOF Hollow Nanospheres for Boosting Oxygen Evolution Reaction Electrocatalysis. Inorg. Chem. 2021, 60, 5882–5889. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wu, Y.; Xue, Q.; Zhu, J.-J.; Zhou, Y. A novel multi-walled carbon nanotube-coupled CoNi MOF composite enhances the oxygen evolution reaction through synergistic effects. J. Mater. Chem. A 2022, 10, 4936–4943. [Google Scholar] [CrossRef]
- Hu, L.; Xiao, R.; Wang, X.; Wang, X.; Wang, C.; Wen, J.; Gu, W.; Zhu, C. MXene-induced electronic optimization of metal-organic framework-derived CoFe LDH nanosheet arrays for efficient oxygen evolution. Appl. Catal. B Environ. 2021, 298, 120599. [Google Scholar] [CrossRef]
- Li, M.; Sun, R.; Li, Y.; Jiang, J.; Xu, W.; Cong, H.; Han, S. The 3D porous “celosia” heterogeneous interface engineering of layered double hydroxide and P-doped molybdenum oxide on MXene promotes overall water-splitting. Chem. Eng. J. 2022, 431, 133941. [Google Scholar] [CrossRef]
- Han, J.; Zhang, J.; Wang, T.; Xiong, Q.; Wang, W.; Cao, L.; Dong, B. Zn Doped FeCo Layered Double Hydroxide Nanoneedle Arrays with Partial Amorphous Phase for Efficient Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 13105–13114. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Hung, S.-F.; Chen, H.-Y.; Chan, T.-S.; Chen, H.M.; Liu, B. In Operando Identification of Geometrical-Site-Dependent Water Oxidation Activity of Spinel Co3O4. J. Am. Chem. Soc. 2015, 138, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xing, C.; Liang, Z.; Guardia, P.; Han, X.; Zuo, Y.; Llorca, J.; Arbiol, J.; Li, J.; Cabot, A. Activating the lattice oxygen oxidation mechanism in amorphous molybdenum cobalt oxide nanosheets for water oxidation. J. Mater. Chem. A 2022, 10, 3659–3666. [Google Scholar] [CrossRef]
- Moysiadou, A.; Lee, S.; Hsu, C.-S.; Chen, H.M.; Hu, X. Mechanism of Oxygen Evolution Catalyzed by Cobalt Oxyhydroxide: Cobalt Superoxide Species as a Key Intermediate and Dioxygen Release as a Rate-Determining Step. J. Am. Chem. Soc. 2020, 142, 11901–11914. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhao, Z.; Wang, J.; Zhou, Q.; Zhao, C.; Yao, Z.; Wang, J. Oxygen-deficient TiO2 and carbon coupling synergistically boost the activity of Ru nanoparticles for the alkaline hydrogen evolution reaction. J. Mater. Chem. A 2021, 9, 10160–10168. [Google Scholar] [CrossRef]
- Kuang, P.; Wang, Y.; Zhu, B.; Xia, F.; Tung, C.-W.; Wu, J.; Chen, H.M.; Yu, J. Pt Single Atoms Supported on N-Doped Mesoporous Hollow Carbon Spheres with Enhanced Electrocatalytic H2-Evolution Activity. Adv. Mater. 2021, 33, 2008599. [Google Scholar] [CrossRef]
- Lv, J.; Liu, P.; Li, R.; Wang, L.; Zhang, K.; Zhou, P.; Huang, X.; Wang, G. Constructing accelerated charge transfer channels along V-Co-Fe via introduction of V into CoFe-layered double hydroxides for overall water splitting. Appl. Catal. B Environ. 2021, 298, 120587. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Liu, J.; Sun, F.; Yang, P.; Qiu, J. A hierarchically porous and hydrophilic 3D nickel–iron/MXene electrode for accelerating oxygen and hydrogen evolution at high current densities. Nano Energy 2019, 63, 103880. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, S.; Wang, Z.; Zhao, J.; Qiu, J. Boosting electrocatalytic oxygen evolution by synergistically coupling layered double hydroxide with MXene. Nano Energy 2018, 44, 181–190. [Google Scholar] [CrossRef]
- Li, D.; Xiang, R.; Yu, F.; Zeng, J.; Zhang, Y.; Zhou, W.; Liao, L.; Zhang, Y.; Tang, D.; Zhou, H. In Situ Regulating Cobalt/Iron Oxide-Oxyhydroxide Exchange by Dynamic Iron Incorporation for Robust Oxygen Evolution at Large Current Density. Adv. Mater. 2023, 36, 2305685. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Yuan, Y.; Zhang, Y.; Lyu, X.; Liu, C.; Yang, X.; Bai, Z.; Wang, H.; Yang, L. Optimizing 3d spin polarization of CoOOH by in situ Mo doping for efficient oxygen evolution reaction. Carbon Energy 2023, 6, e418. [Google Scholar] [CrossRef]
- Zhu, Y.; An, S.; Sun, X.; Lan, D.; Cui, J.; Zhang, Y.; He, W. Core-branched NiCo2S4@CoNi-LDH heterostructure as advanced electrode with superior energy storage performance. Chem. Eng. J. 2020, 383, 123206. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, X.; He, L.; Zheng, Y.; Pang, J.; Wang, L.; Jiang, R.; Hou, J.; Guo, X.; Chen, L. Structural and Electronic Modulation of Iron-Based Bimetallic Metal–Organic Framework Bifunctional Electrocatalysts for Efficient Overall Water Splitting in Alkaline and Seawater Environment. ACS Appl. Mater. Interfaces 2022, 14, 46374–46385. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.-J.; Chen, H.; Li, Y.-N.; Shao, L.; Ma, J.-C.; Li, L.-L.; Chen, J.-Y.; Wang, T.-Q.; Zhang, X.-M.; Zhang, L.-Y.; et al. CoNi-based metal–organic framework nanoarrays supported on carbon cloth as bifunctional electrocatalysts for efficient water-splitting. New J. Chem. 2020, 44, 1694–1698. [Google Scholar] [CrossRef]
- Xu, H.; Fei, B.; Cai, G.; Ha, Y.; Liu, J.; Jia, H.; Zhang, J.; Liu, M.; Wu, R. Boronization-Induced Ultrathin 2D Nanosheets with Abundant Crystalline–Amorphous Phase Boundary Supported on Nickel Foam toward Efficient Water Splitting. Adv. Energy Mater. 2020, 10, 1902714. [Google Scholar] [CrossRef]
- Raja, D.S.; Chuah, X.-F.; Lu, S.-Y. In Situ Grown Bimetallic MOF-Based Composite as Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting with Ultrastability at High Current Densities. Adv. Energy Mater. 2018, 8, 1801065. [Google Scholar] [CrossRef]
- Chen, C.; Suo, N.; Han, X.; He, X.; Dou, Z.; Lin, Z.; Cui, L. Tuning the morphology and electron structure of metal-organic framework-74 as bifunctional electrocatalyst for OER and HER using bimetallic collaboration strategy. J. Alloys Compd. 2021, 865, 158795. [Google Scholar] [CrossRef]
- Mou, Q.; Xu, Z.; Wang, G.; Li, E.; Liu, J.; Zhao, P.; Liu, X.; Li, H.; Cheng, G. A bimetal hierarchical layer structure MOF grown on Ni foam as a bifunctional catalyst for the OER and HER. Inorg. Chem. Front. 2021, 8, 2889–2899. [Google Scholar] [CrossRef]
- Chai, N.; Kong, Y.; Liu, T.; Ying, S.; Jiang, Q.; Yi, F.-Y. (FeMnCe)-co-doped MOF-74 with significantly improved performance for overall water splitting. Dalton Trans. 2023, 52, 11601–11610. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Zhang, J.; Hu, Y.; Wang, L.; Zhang, X.; Zhao, B. Ni Doped Co-MOF-74 Synergized with 2D Ti3C2Tx MXene as an Efficient Electrocatalyst for Overall Water-Splitting. Catalysts 2024, 14, 184. https://doi.org/10.3390/catal14030184
Yu K, Zhang J, Hu Y, Wang L, Zhang X, Zhao B. Ni Doped Co-MOF-74 Synergized with 2D Ti3C2Tx MXene as an Efficient Electrocatalyst for Overall Water-Splitting. Catalysts. 2024; 14(3):184. https://doi.org/10.3390/catal14030184
Chicago/Turabian StyleYu, Ke, Jingyuan Zhang, Yuting Hu, Lanqi Wang, Xiaofeng Zhang, and Bin Zhao. 2024. "Ni Doped Co-MOF-74 Synergized with 2D Ti3C2Tx MXene as an Efficient Electrocatalyst for Overall Water-Splitting" Catalysts 14, no. 3: 184. https://doi.org/10.3390/catal14030184